Publication History
Submitted: April 05, 2025
Accepted: April 20, 2025
Published: May 31, 2025
Identification
D-0423
DOI
https://doi.org/10.71017/djmi.4.5.d-0423
Citation
Sharif Masuma Ismat, Chowdhury Shamima Sultana, Maksuda Begum & Latifa Akhter (2025). Efficacy of FNAC in the Diagnosis of Advanced Ovarian Malignancy. Dinkum Journal of Medical Innovations, 4(05):230-244.
Copyright
© 2025 The Author(s).
230-244
Efficacy of FNAC in the Diagnosis of Advanced Ovarian MalignancyOriginal Article
Sharif Masuma Ismat 1*, Chowdhury Shamima Sultana 2, Maksuda Begum 3, Latifa Akhter 4
- Consultant, Gynae & Obs., 250 Beded Sadar Hospital Brahmanbaria, Bangladesh.
- Associate Professor, Gynaecological Oncology, National Institute of Cancer Research & Hospital, Dhaka, Bangladesh.
- Jr.Consultant, Gynae & Obs., Upozila Health Complex ,Chokoria Cox Bazar, Bangladesh.
- Associate Professor, Gynaecological Oncology Department, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh.
* Correspondence: smismat@gmail.com
Abstract: Worldwide, 6,044,000 women are diagnosed with cancer and 3,345,000 die from the disease each year. From 1975–2010, the age-standardized incidence rates for cancers in women have increased by 42%. Ovarian cancer accounts for 4% of all female cancers and 31% of cancers of the female genital tract, with more than 190,000 new cases diagnosed worldwide each year. Ovarian cancer is the fourth most common cause of death from malignancy in women This study assessed the diagnostic value of fine needle aspiration cytology (FNAC) in advanced ovarian malignant lesions and correlated FNAC result with Histopathologic findings of surgical specimen, and found out sensitivity, specificity, positive predictive value, negative predictive value of FNAC as a diagnostic test in malignant ovarian tumor. The study is conducted at Inpatient and outpatient department of Gynecological Oncology, Oncology, Pathology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh for a Study period of one year. Cross sectional analytical type of study design was used Main outcome variables to be studied are the cytological report of FNAC and the histopathological report of specimens after debulking surgery. When combined with radiological assessment of the nature of the tumor, FNAC can serve as a highly efficient means of diagnosis of advanced ovarian neoplasms. In the study cases common clinical presentation were pain, distension of abdomen, tenderness, anorexia, nausea and early satiety. Majority had presented with lump size were < 100 cm2, most of the lump were in irregular margins, surfaces were nodular, consistency were solid and partly solid and partly cystic and. Mobility were restricted in majority cases and minority were freely mobile. Majority of serum CA 125 level were > 300 mI U/ml. Maximum FNAC findings of ovary was Adeno Carcinoma and maximum histological types was Papillary Serous Cyst adenocarcinoma and Serous Cyst adenocarcinoma. Performance of diagnostic test sensitivity of FNAC findings was 96.43%, specificity 100%, accuracy 96.67%, positive and negative predictive values were 100% and 66.67% respectively.
Keywords: gynecological oncology, FNAC, carcinoma, pathology, diagnostic
- INTRODUCTION
Worldwide, 6,044,000 women are diagnosed with cancer and 3,345,000 die from the disease each year [1]. From 1975–2010, the age-standardized incidence rates for cancers in women have increased by 42% [2]. Ovarian cancer accounts for 4% of all female cancers and 31% of cancers of the female genital tract, with more than 190,000 new cases diagnosed worldwide each year. Ovarian cancer is the fourth most common cause of death from malignancy in women [1]. A woman’s risk at birth of having ovarian cancer some time in her lifetime is nearly 1.4%, and the risk of dying from ovarian cancer is almost 1%. The number of new cases per 10,000 women per year was 12.5 in 2010. It is the fifth most common cancer in women in the United States after cancers of the lung, breast, colon, and uterus. There is a trend toward improved survival for ovarian cancer [1,2]. Based on Surveillance, Epidemiology, and End Results (SEER) data in the United States ovarian cancer rates are highest in women aged 55 to 64 years (median age 63 years), The surgical management of all patients with advanced-stage disease is approached in a similar manner, with modifications made for the overall status and general health of the patient. If the patient is medically stable, she should undergo an initial exploratory procedure with removal of as much disease as possible [3,4]. The operation to remove the primary tumor as well as the associated metastatic disease is referred to as debulking or cytoreductive surgery. In selected patients who are not candidates for initial cytoreductive surgery, neoadjuvant chemotherapy may be given for a few cycles before surgery. The preoperative assessment of resect ability is limited. Using a cutoff of 500 International Unit, CA125 levels have been suggested as a means of predicting the probability of an optimal resection, but others have shown that these determinations have low predictive value [5,6,7]. CT and MRI scans have been used to try to predict suboptimal resection [8,9,10]. In a series from the Mayo Clinic [8], the presence of diffuse peritoneal thickening and ascites on CT scan was associated with a 32% optimal debulking rate, as opposed 71% in the group that did not have these findings, with a positive predictive value of 68%. However, in a larger multi-institutional validation study, the accuracy of CT in predicting suboptimal cytoreduction dropped to as low as 34% in some cohorts [11]. CT–PET also has limited positive predictive value [12,13]. Vergote and colleagues from Belgium reported the use of open laparoscopy in 173 patients with a pelvic mass, an omental “cake,” or large volume ascites to exclude other primary tumors and to determine resect ability. Seventy-one of the patients (41%) developed a port site metastasis [14]. In experience, it is almost always possible to remove the primary tumor (if necessary, with an en bloc resection of the recto-sigmoid colon) and the omental cake, so the major reason for recommending neo-adjuvant chemotherapy is to improve the medical fitness of the patient. Neoadjuvant chemotherapy is a viable approach for the limited number of patients felt to be optimally unresectable by an experienced ovarian cancer surgical team, that is, in selected patients who are at high risk for operative morbidity or mortality [15,16]. As discussed above, primary cytoreductive surgery should be considered the standard of care for most patients. There may be a role for neoadjuvant chemotherapy in selected patients with stage III or stage IV ovarian cancer with large-volume disease with extensive ascites and large pleural effusions as well as in patients who have a poor performance status and are therefore at high operative risk because of medical comorbidities [17]. This highlighted the need for preoperative cytological diagnosis of ovarian tumors. An author [18] reported the superiority for histology and/or cytology over clinical factors (CAl25 and radiology) for diagnosis of epithelial ovarian tumors prior to neo-adjuvant chemotherapy. An author [19], also concluded from their study that ultrasound and CT guided Fine Needle Aspiration Cytology (FNAC) can be an optimum modality for the diagnosis of primary and metastatic ovarian neoplasm and evaluation of recurrent malignant tumors, which has great impact on patient management consequently. Considering FNAC adequacy; [20] reported that it was (83.3%), [21] reported (86%), [22] reported (75%), and [23] reported (77%) while [24] reported a higher value (97.1%). Higher values for FNAC adequacy reported by [24] may be due to the presence of cytopathologist and cytotechnologist attending the procedure for all studied population. An author [25] reported overall diagnostic accuracy in malignant category is 88.2%. In the study population is suspected malignant case so it was the desired efficacy of study. Ovarian cancer is a silent killer; however, improvements in identification of women at high risk for ovarian cancer, as well as improved imaging techniques, increase the likelihood of early detection [26,27,28]. Clinical examinations then correlation with pelvic ultrasonography is the examination of choice, followed by magnetic resonance imaging (MRI) and/or computed tomography (CT) scanning [29,30,31]. The ovary may be difficult to delineate in some women who are postmenopausal, because of its relatively small size (<2 × 2 cm), its position deep within the pelvis, and the lack of identifiable contained structures, such as cysts [32,33]. Relatively simple ultrasound-based rules can be used to diagnose ovarian malignancy, such as the International Ovarian Tumor Analysis (IOTA) rules [27,28].A multicenter trial [27] evaluated 1938 patients with an adnexal mass: 1396 (72%) had benign tumors, 373 (19.2%) had primary invasive tumors, 111 (5.7%) had borderline malignant tumors, and 58 (3%) had metastatic tumors in the ovary. Five simple (M) rules were used to predict malignancy: irregular solid tumor; ascites; at least four papillary structures; irregular multilocular-solid tumor with a largest diameter of at least 100 mm; and very high color content on color Doppler examination. Five simple (B) rules were used to suggest a benign tumor: unilocular cyst; presence of solid components where the largest solid component is <7 mm in largest diameter; acoustic shadows; smooth multilocular tumor less than 100 mm in largest diameter; and no detectable blood flow on Doppler examination. If one or more M features were present in the absence of a B feature, the mass was classified as malignant. If one or more B features were present in the absence of an M feature, it was classified as benign. If both M features and B features were present, or if none of the features was present, the simple rules were inconclusive. The simple rules yielded a conclusive result in 1501 masses (77%), for which they resulted in a sensitivity of 92% (95% confidence interval 89% to 94%) and a specificity of 96% (94% to 97%). Malignant ovarian tumors tend to have papillary excrescences, irregular walls, and/or thick septations [34,35]. The tumor can contain echogenic material arising from mucin or protein debris. The more solid the areas are, the greater the likelihood that a tumor is present. Typically, intraperitoneal fluid is present; this is a sign of peritoneal spread. On color Doppler ultrasonograms, tumors tend to have vessels with low impedance because of the lack of muscular media in the vessel wall and arteriovenous shunts. The vessels tend to be clustered. The ultrasonographic finding that is most indicative of ovarian cancer is papillary excrescence, which is present in more than 50% of ovarian malignancies. Low impedance and clustered vessels have a 70-80% diagnostic accuracy [31]. The primary use of CT scanning is in the evaluation of metastatic disease rather than of the ovarian mass; for the evaluation of the ovarian mass, ultrasonography and MRI are more valuable [26]. CT scanning is helpful in diagnosing cystic teratomas, 93% of which contain fat and 56% of which are calcified. If a large (>10 cm) soft-tissue mass is present, malignant transformation should be suspected [36]. CT scanning also can aid in the evaluation of cyst adenocarcinomas. A serous cyst adenocarcinoma has an attenuation similar to that of water, whereas a mucinous cyst adenocarcinoma has an attenuation closer to that of soft tissue. The presence of wall and septal thickness and irregularity, as well as the existence of enhancing nodules, suggests malignancy. Although CT scan findings can suggest malignancy, they are not definitive for diagnosis unless metastases are present. With the availability of modern techniques, USG and CT guided FNAC can be an optimum modality for research and diagnosis of primary and metastatic ovarian neoplasm and evaluation of recurrent malignant tumors, which has great impact on patient management consequently. Ovarian cancer is the eighth most common cancer in woman and the deadliest in terms of absolute number and the majority of the patients present with advanced disease, making prognosis poor. There is clear association between the stages and prognosis of ovarian malignant tumors. Since two-thirds of epithelial ovarian cancer cases present at advanced stages and have a low 5-year survival rate, early evaluation of ovarian lesions is very important. Surgery for these groups of patients is incomplete and optimal debulking is often not possible. Even if it is done with difficulty, post operative morbidity is very high. Introduction of neoadjuvant chemotherapy followed by interval debulking is found to improve the cyto-reduction and reduce surgery related morbidity in such advanced cases. In addition, neo-adjuvant chemotherapy is now gaining popularity in improving physical and emotional trauma associated with initial surgery. This highlighted the need for preoperative cytological diagnosis of ovarian tumors. Cytology has been underutilized as a modality for the diagnosis of ovarian tumors. With the advent of accurate imaging techniques like ultrasonography (USG) and computed tomography (CT) scan in detecting the ovarian lesions and omental or peritoneal deposit, guided fine needle aspiration cytology (FNAC) has assumed a definite role in diagnosis and management. So, efficacy of FNAC is an important issue for the diagnosis and management purpose of advanced ovarian malignancy. This study assessed the diagnostic value of fine needle aspiration cytology (FNAC) in advanced ovarian malignant lesions and correlated FNAC result with Histopathologic findings of surgical specimen, and found out sensitivity, specificity, positive predictive value, negative predictive value of FNAC as a diagnostic test in malignant ovarian tumor.
- MATERIALS & METHOD
The study is conducted at inpatient and outpatient department of Gynecological Oncology, Oncology, Pathology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh for a Study period of one year. Cross sectional analytical type of study design was used Main outcome variables to be studied are the cytological report of FNAC and the histopathological report of specimens after debulking surgery. Study population included all the patients with advanced ovarian malignancy attended in outpatient and inpatient department of Gynecological Oncology, Oncology, pathology. Sample size determination depends on time and resources. As prevalence of advanced ovarian malignancy are not known in our country, so estimated population was calculated by using the following statistical formula:
n=z2p (1-p)/d2
Where n= the desired sample size
Z=the standard normal deviate, usually set at 1.96 at 5 % level which corresponds to 95% confidence level.
P means prevalence = 0.5 (50%), (In unknown prevalence it can be regarded as 50%). The degree of accuracy or precision level is d which is considered at 10%. The higher value of d was yielding lower sample size and smaller value of d was yield higher sample size. Suppose 50% (p=0.5) of the admitted patients have advanced ovarian malignancy. Z statistic is 1.96, which corresponds to the 95% confidence level. d is the level of accuracy that is considered 10%. Using the above formula the expected sample size was n=96. But 30 cases were included in this study due to timer limitation of study period. Sampling was done by Convenient sampling technique. Advanced ovarian malignancy-Stage iii and iv diseases are suspected as a case of advanced in case of ovarian malignancy. In stage iii disease confined to one or both ovaries with extra pelvic peritoneal metastases and stage iv is distant metastases and extra abdominal organ involvement. Detailed history of the patient who attended in the Department of Gynecological Oncology, Oncology, Pathology through outpatient and inpatient department, with lump in lower abdomen had been taken. Therefore, through clinical examination was performed and some laboratory investigations were sent. Clinically or radiologically suspected as advanced ovarian malignancy such as patient with‑ Pleural effusion, Liver metastases, Enlarged supraclavicular lymph node, Hard, fixed irregular lump or large volume tumor, Epigastric lump with or without extensive ascites. The patients who were not fit for primary surgery were selected for FNAC. If the FNAC reports were positive for malignancy were selected for neoadjuvant chemotherapy. After completion of neo-adjuvant chemotherapy interval debulking was done. The patients who were negative underwent laparotomy. Tissues removed by surgery were sent for histopathological evaluation. Cytology results were compared with histopathological diagnosis. The data were analyzed by statistical (SPSS) method and were present in the form of tables, figures, graphs, diagrams and other charts etc. Questionnaires were filled up and data are collected on data collection sheet including the all variables of interest. Data were processed and analyzed using SPSS (Statistical Package for Social Sciences) software version 20. The chi- square test and student “t” test was used to analyze the significance level of p < 0.05. Continuous scale data were presented as mean standard deviation and Categorical data were presented as number percentage. The summarize data were present in the table and chart. Institutional permission to collect data was taken before conducting the study. Informed verbal consents were obtained from the patient or parties. The study was not interfered with management or deal with moral issues.
- RESULTS & DISCUSSION
Table 01: Age group distribution of the study population
| Number | Percentage | |
| 31-40 yrs | 06 | 20.0 |
| 41-50 yrs | 10 | 33.3 |
| 51-60 yrs | 11 | 36.7 |
| > 60 yrs | 03 | 10.0 |
| Total | 30 | 100.0 |
| Mean ±SD | 50.96(±9.03) | Range 32-65 years |
Table shows mean age was 50.96(±9.03) years, minimum age was 32 years and maximum age was 65 years. Table shows majority of the respondent came from middle class socio-economic status.
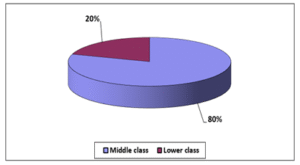
Figure 01: Socio-economic status of the study population
Table 02: Distribution of parity of the study population
| Parity | Number | Percentage |
| 1-2 | 13 | 43.3 |
| 3-5 | 13 | 43.3 |
| > 5 | 04 | 13.3 |
| Total | 30 | 100.0 |
Table shows 43.3% mother had parity between 1-2,13(43.3%) mother had parity 3-5 and 04(13.3%) had parity >5.
Table 03: Menopausal status of the study population
| Menopause status | Number | Percentage |
| Pre-menopause | 14 | 46.7 |
| Post menopause | 16 | 53.3 |
| Total | 30 | 100 |
Table shows majority 14(46.7%) were pre-menopausal and 16(53.3%) were postmenopausal.
Table 04: Age of menarche of the study population
| Number | Percentage | |
| ≤13 years | 18 | 60 |
| > 13 years | 12 | 40 |
| Total | 30 | 100 |
Table shows age of menarche of the study population, ≤13 years in case of 18(60%) cases and > 13 years in cases 12(40%). Figure age of menopause of the study population, < 50 years in case of 9(56.2%) cases and ≥ 50 years in cases 7(43.75%).
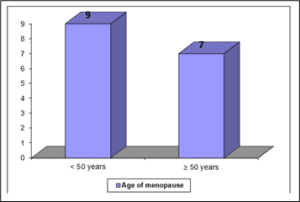
Figure 02: Age of menopause of the study population
Table 05: Uses of OCP as a method of contraception of the study population
| Number | Percentage | |
| OCP user | 06 | 20.0 |
| OCP non user | 24 | 80.0 |
| Total | 30 | 100 |
Table shows 06(20%) were OCP user and 24(80%) were OCP non user.
Table 06: Anemic status of the study population
| Anemia | Number | Percentage |
| Mild | 16 | 53.3 |
| Moderate | 11 | 36.7 |
| Non anemic | 03 | 10.0 |
| Total | 30 | 100.0 |
Table shows majority 16(53.3%) were mild anemic, 11(36.7%) were moderate anemic and 03(10%) were non anemic.
Table 07: Clinical presentation of the study population
| Number | Percentage | |
| Pain | 20 | 66.67 |
| Distension of abdomen | 15 | 50.00 |
| Tenderness | 25 | 83.33 |
| Anorexia | 10 | 33.33 |
| Nausea | 12 | 40.00 |
| Early satiety | 18 | 60.00 |
Table shows common clinical presentation were pain, distension of abdomen, tenderness, anorexia, nausea and early satiety which were 66.7%, 50%, 83.3%, 33.3%, 40% and 60% respectively.
Table 08: Associated condition of the study population
| Number | Percentage | |
| Engorged vessels | 06 | 20.00 |
| Ascites | 23 | 76.67 |
| Pleural effusion | 14 | 46.67 |
Table shows 06(20%) presented with engorged vessels, 23(76.7%) with ascites and 14(46.67%) with pleural effusion.
Table 9: Clinical examination findings of the lump of study population
| LUMP | Number | Percentage |
| · Present | 18 | 60.0 |
| · Absent | 12 | 40.0 |
| Size of lump | ||
| · < 100 cm2 | 13 | 72.2 |
| · ≥ 100 cm2 | 05 | 27.8 |
| Margin | ||
| · Regular | 04 | 22.2 |
| · Irregular | 14 | 77.8 |
| Surface | ||
| · Smooth | 5 | 27.8 |
| · Nodular | 13 | 72.2 |
| Consistency | ||
| · Partly Solid Partly Cystic | 08 | 44.4 |
| · Solid | 10 | 56.6 |
| Mobility | ||
| · Mobile | 07 | 38.9 |
| · Restricted | 11 | 61.1 |
Table shows Clinical examination findings of the lump of study population 18(60%) had presented with lump. Size of majority 13 (72.2%) of lump were < 100 cm2, margins were irregular in 14 (77.8%) cases. Surfaces were nodular in 13 (72.2%) cases and 05 (27.8%) cases were smooth, consistency were partly solid and partly cystic in 10 (56.6%) cases and only solid in 07 (38.9%) cases. Mobility was restricted in (61.1%) and 07 (38.9%) were freely mobile.
Table 10: Serum CA 125 level of the study population
| CA 125 | Number | Percentage |
| · ≤ 35 | 02 | 6.7 |
| · 35-300 | 05 | 16.7 |
| · > 300 | 23 | 76.7 |
| Total | 30 | 100.0 |
Table shows serum CA 125 level 02(6.7%) were ≤ 35 mI U/ml 05(16.7%) were 35-300 mI U/ml and 23(76.7%) were > 300 mI U/ml.
Table 11: Serum CA 19-9 level of the study population
| CA 19-9 | Number | Percentage |
| < 37 | 11 | 84.6 |
| ≥ 37 | 02 | 15.4 |
| Total | 13 | 100.0 |
Table shows serum CA 19-9 level 11(84.6%) were <37 mI U/ml and 02(15.4%) were ≥37 mI U/ml.
Table 12: Albumin level of the study population
| Albumin level | Number | Percentage |
| < 30 | 19 | 63.3 |
| ≥ 30 | 11 | 36.7 |
| Total | 30 | 100.0 |
Table shows albumin level 19(63.3%) were <30 and 11(36.7%) were ≥30.
Table 13: FNAC findings of the study population
| FNAC findings | Number | Percentage | |
| Ovary | |||
| Adeno Carcienoma | 15 | 50.0 | |
| Malignant Cell Present | 05 | 16.7 | |
| Mucinous Cyst adenocarcinoma | 01 | 3.3 | |
| Papillary Serous Cyst adenocarcinoma | 01 | 3.3 | |
| Malignant Cell Absent | 03 | 6.7 | |
| Pleural Fluid | |||
| Malignant cell present | 01 | 3.3 | |
| Ascitic fluid | Metastatic adeno carcinoma | 02 | 6.7 |
| Scalaenae | Metastatic adeno carcinoma | 01 | 3.3 |
| Metastatic deposited | |||
| Metastatic Serous Adeno Carcinoma | 01 | 3.3 | |
| Malignant Cell Present | 02 | 6.7 | |
| Omen tum | Metastatic Adenocarcinoma | 01 | 3.3 |
Table shows FNAC findings in ovary 15(50%) were Adenocarcinoma, 05(16.7%) were malignant Cell Present, 1(3.3%) were Mucinous Cyst adenocarcinoma and Papillary Serous Cyst adenocarcinoma each. 01 (3.3%) malignant cell present was found from pleural fluid, 02(6.7%) metastatic adeno carcinoma were found from ascitic fluid, 01(3.3%) metastatic adeno carcinoma found from scalaenae, 01(3.3%) metastatic serous adeno carcinoma and 2(6.7%) malignant cells present from metastatic deposited, 1(3.3%) metastatic adeno carcinoma from omen tum.
Table 14: Histological types of malignant ovarian tumor of the study population
| Histological type | Number | Percentage |
| Papillary Serous Cyst adenocarcinoma | 11 | 36.67 |
| Serous Cyst adenocarcinoma | 08 | 26.67 |
| High Grade Serous Adeno Carcinoma | 02 | 6.67 |
| Mucinous Cyst adenocarcinoma | 03 | 10.00 |
| Secondary Metastatic Ductal Carcinoma | 01 | 3.33 |
| Endometroid Adenocarcinoma | 04 | 13.33 |
| Tuberculosis | 01 | 3.33 |
| Total | 30 | 36.67 |
Table shows histological types Papillary Serous Cyst adenocarcinoma were 11(36.67%), Serous Cyst adenocarcinoma was 08(26.67%), High Grade Serous Adeno Carcinoma 02(6.7%), Mucinous Cyst adenocarcinoma was 3(10%), Secondary Metastatic Ductal Carcinoma was 1(3.3%), Endometroid Adeno Carcinoma 04(13.3%) and Tuberculosis was 01(3.3%)
Table 15: Relation between FNAC findings with histopathology findings
| FNAC | Histopathology | Total | P value | |
| Malignant | Malignant cell absents | |||
| Malignant | 27 | 00 | 27 | 0.007 |
| Malignant cell absents | 01 | 02 | 03 | |
| Total | 28 | 02 | 30 | |
Table 16: Performance of diagnostic test
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| FNAC vs Histopathology | 96.43% | 100% | 100% | 66.67% | 96.67% |
Table shows that sensitivity of FNAC findings was 96.43%, specificity 100%, accuracy 96.67%, positive and negative predictive values were 100% and 66.67% respectively.
DISCUSSION
In this study showed mean age was 50.96(±9.03) years, minimum age was 32 years and maximum age was 65 years. More than half the cases in Ray et al. series belonged to 21 to 40 years age group, as also the experience of other researchers [37]. In study of [38] the mean age of our cases was 52.7 years, (range: 11-63 years) this result is similar our study. In a study [39] Patients age ranged from 10-80 years with a maximum no of cases (26.70%) in 41–50-year age group, the mean age being 44 years. In present study majority 53.3% were pre-menopausal and 14(46.7%) were post-menopausal. (43.3%) mother had parity between 1-2,13(43.3%) mother had parity 3-5 and 04(13.3%) had parity >5. Majority 16(53.3%) were mild anemic, 11(36.7%) were moderate anemic and 03(10%) were non anemic. Common clinical presentation was pain, distension of abdomen, tenderness, anorexia, nausea and early satiety which were 66.7%, 50%, 83.3%, 33.3%, 40% and 60% respectively. Majority18 (60%) of the patient presented with lump. Size of lump were < 100 cm2 in 13 (72.2%) cases. Margin were irregular 14(77.8%), 13(72.2%) presented with nodular and 05(27.8%) with smooth surface. Consistency was partly solid and partly cystic in 08 (44.4%) cases and 07(38.9%) were solid, mobility was restricted in11(61.1%) cases and 07(38.9%) were freely mobile. A study [40] showed among the symptomatic cases; pain abdomen was the commonest symptom (91.04%) followed by menstrual irregularities (23.9%) and abdominal palpable lump (20.9%). Similar experiences were also published previously [25, 41,42]. FNAC findings were 15(50%) Adenocarcinoma, 05(16.7%) malignant cell present, 1(3.3% Mucinous Cyst adenocarcinoma and Papillary Serous Cyst adenocarcinoma each. In metastatic cases malignant cell present was found from pleural fluid in 1(3.3%) case, 02(6.7%) metastatic adeno carcinoma were found from ascitic fluid, 01(3.3%) metastatic adeno carcinoma found from scalaenae, 01(3.3%) metastatic serous adeno carcinoma and 2(6.7%) malignant cells present from metastatic deposited, 1(3.3%) metastatic adeno carcinoma from omen tum. A study [43] FNAC findings Serous cystadenocarcinoma 7 cases, Mucinous cystadenocarcinoma 2 cases, Granulosa cell tumor 2 cases, Dysgerminoma 2 cases. In a study [44] Papillary cystadenoma, Cystadenocarcinoma, Endometrioid Carcinoma, Metastatic adenocarcinoma. An author [45] Aspirates from both cases were scanty fluid containing macrophages, degenerated cells and few round cells in small clusters having scanty to moderate cytoplasm in a clear background. The smears were interpreted as non-neoplastic cysts-possibly follicular cyst, according to criteria reported by [46]. Histopathological examination of both the cysts established diagnosis of serous cystadenoma. Possible cause of cytological misdiagnosis was scanty cellular materials from degenerated atrophic lining epithelium. Similar experiences were reported by a lot of other workers [47,48]. In study [49] , we were able to diagnose papillary malignant tumors accurately on both cytological and histopathological examinations. Our study was also supported by Vijayakumar [50] who demonstrated 100% correlation between cyto-/histological findings of papillary cystadenoma and papillary cyst adenocarcinoma. On examining the germ cell tumors, 9 cases of granulosa cell tumors, 3 cases of dysgerminoma and 6 cases of mature teratoma was accurately diagnosed by cytological examination. Our findings were supported by [51] and [52], who also found 100% diagnostic accuracy of cytological examination in detecting epithelial cell tumors. In our study, endometrioid carcinoma was observed to be cytologically similar to serous tumors but we had successfully correlated our four cases of endometrioid carcinoma. In this study showed that sensitivity of FNAC findings was 96.43%, specificity 100%, accuracy 96.67%, positive and negative predictive values were 100% and 66.67% respectively. All but one of the non-neoplastic cystic lesions were diagnosed accurately by FNAC, which included 2 cases reported as possibly benign and 5 more cases where histopathology was not performed since the cyst dimension was less than or equal to 5 cm [53]. These latter 5 cases of follicular cyst were diagnosed as ‘benign cystic lesion’ on cytology. One endometriotic cyst was erroneously diagnosed as a serous carcinoma, while the remaining 6 endometriotic cysts were diagnosed accurately. Another case where histology was not available was a serous cystadenocarcinoma with metastasis and peritoneal nodules. For all 83 cases, the sensitivity and specificity of FNAC considering final (histological) diagnosis as the gold standard were 83% and 97%, respectively, with a diagnostic accuracy of 93%. Chi square test was performed to correlate between cytological and final (histological) diagnosis, and was highly significant (p<0.001). an author [54] also reported that the majority of cystic ovarian lesions can be diagnosed accurately; however, they did not correlate FNAC with histology in 53% of their cases [55]. Aysun & Canan compared the findings of FNAC and histology in ovarian masses and found a high sensitivity (95.1%) and a specificity of 90.4% [56]. Gupta and Rajvanshi found a sensitivity of 85.7% and a specificity of 98.0% [57]. Cole and co-workers found FNAC to be highly specific (100%) but conversely with a very low sensitivity of only 50% [58]. Our observations corroborate closely with those of other investigators, which indicates that FNAC can have appreciable sensitivity, specificity and accuracy in the diagnosis of ovarian masses. An author [59] reported a specificity of 90% in the cytological evaluation of ovarian cysts, which is comparable to the present study, but showed a much lower sensitivity of 25%. This may have been due to the inclusion of cystic ovarian lesions only in their study and the aspiration of cysts in post-surgical specimens Only [18], and [50] discussed the accuracy of FNAC for tumor typing similar to our study. In both studies ability for estimation of tumor type (83.8% for [50] and 55% for Freedman et al.) was higher than that in our study (39.4%). This may be due to the fact that most of [55] cases were surface epithelial tumors mostly high-grade serous carcinomas. Typing on FNAC is mostly difficult in high grade tumors when compared to low grade ones. Differentiation of serous from endometroid carcinomas may be impossible on FNAC. An author [52] who found the marked reduction in the number of inadequate samples to 4% when both techniques were combined. However, there wasn’t significant change as regard diagnostic accuracy, sensitivity, specificity, PPV or NPV also similar to [22] and [60]. The sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy were 96.43, 100, 100, 66.67 and 96.67, respectively in our study. An author [60] in their cytological and histological ovarian tumors correlated study observed sensitivity, specificity and diagnostic accuracy of 95.8, 96.0 and 95.8% respectively. However, they did not observe the positive and negative predictive values in their study. They also observed 2% of the results as inconclusive, however, no inconclusive results were observed in our cases. In a study conducted by [61] between malignant/borderline/benign ovarian tumors they observed the sensitivity, specificity and diagnostic accuracy as 89.5, 90.3 and 83.6% respectively. An author [41] compared the benign and malignant ovarian cysts by aspiration cytology and observed the sensitivity (75%), specificity (100%) and diagnostic overall accuracy (96%) in their study. An author [62] observed a sensitivity, specificity and diagnostic accuracy of 90, 85, and 93-95% in their study on ovarian tumors by fine needle aspiration biopsy.
- CONCLUSION
In cases of malignant tumors, FNAC has a definitive role in evaluating patients with malignant ovarian tumor, suspected recurrence of the tumor and to assess spread of the disease. However, its use as a first-line diagnostic modality is debatable. Although there is no doubt about the accuracy of diagnosis, the major drawback is that FNAC can lead to rupture and spillage of tumor cells into the peritoneal cavity and can potentially cause upstaging of a malignant tumor. It has been seen that USG and CT can help in identifying tumors as benign or malignant This can serve as a useful guide in assessing which tumors should be aspirated and which should be taken up for surgical evaluation. Despite the potential disadvantages, image-guided FNAC has an important role to play in the diagnosis and management of most ovarian masses. When combined with radiological assessment of the nature of the tumor, FNAC can serve as a highly efficient means of diagnosis of advanced ovarian neoplasms. In study cases common clinical presentation were pain, distension of abdomen, tenderness, anorexia, nausea and early satiety. Majority had presented with lump size were < 100 cm2, most of the lump were in irregular margins, surfaces were nodular, consistency were solid and partly solid and partly cystic and. Mobility were restricted in majority cases and minority were freely mobile. Majority of serum CA 125 level were > 300 mIU/ml. Maximum FNAC findings of ovary was Adeno Carcinoma and maximum histological types was Papillary Serous Cyst adenocarcinoma and Serous Cyst adenocarcinoma. Performance of diagnostic test sensitivity of FNAC findings was 96.43%, specificity 100%, accuracy 96.67%, positive and negative predictive values were 100% and 66.67% respectively. According to selection criteria it has been selected suspected advanced ovarian malignant cases. So, it may be concluded that the role of FNAC in the diagnosis of advanced ovarian malignancy is no more debatable.
REFERENCES
- Estimated Incidence, Mortality and Prevalence Worldwide in 2008;All Cancers (excluding non-melanoma skin cancer) 2008. Available from: http://globocan.iarc.fr/factsheet.asp, Accessed October 20, 2013
- Cancer incidence for all cancers combined 2013. Available from: http://www.cancerresearchuk. org/cancer-info/ cancerstats/ incidence/ all-cancers-combined/, Accessed October 11, 2013
- Anupama Sharma, Dr. Himanshu Shah & Dr. Vandana Mourya (2024). The evaluation of maternal morbidity and perinatal morbidity & mortality in Breech Delivery and Its Comparison with Mode of Delivery. Dinkum Journal of Medical Innovations, 3(02):89-101.
- Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20(5):1260–1268
- Anderson CK, Wallace S, Guiahi M, et al. Risk-reducing salpingectomy as preventative strategy for pelvic serous cancer. Int J Gynecol Cancer. 2013;23(3):417–421
- Muto MG, Cramer DW, Brown DL, et al. Screening for ovarian cancer: The preliminary experience of a familial ovarian cancer center. Gynecol Oncol. 1993;51(1):12–20
- Schwartz PE, Chambers JT, Taylor KJ. Early detection and screening for ovarian cancer. J Cell Biochem Suppl. 1995;23: 233–237
- Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20(5):1260–1268
- Liede A, Karlan BY, Baldwin RL, et al. Cancer incidence in a population of Jewish women at risk of ovarian cancer. J Clin Oncol. 2002;20(6):1570–1577
- Oei AL, Massuger LF, Bulten J, et al. Surveillance of women at high risk for hereditary ovarian cancer is inefficient. Br J Cancer. 2006; 94(6):814–819
- Fries MH, Hailey BJ, Flanagan J, et al. Outcome of five years of accelerated surveillance in patients at high risk for inherited breast/ ovarian cancer: Report of a phase II trial. Mil Med. 2004;169(6): 411–416.
- Meeuwissen PA, Seynaeve C, Brekelmans CT, et al. Outcome of surveillance and prophylactic salpingo-oophorectomy in asymptomatic women at high risk for ovarian cancer. Gynecol Oncol. 2005; 97(2):476–482.
- Oei AL, Massuger LF, Bulten J, et al. Surveillance of women at high risk for hereditary ovarian cancer is inefficient. Br J Cancer. 2006; 94(6):814–819
- Ashmita Pathak (2024). Association of Serum Lactate Dehydrogenase Level with Maternal & Fetal Outcome in Women with Pregnancy Induced Hypertension at BPKIHS. Dinkum Journal of Medical Innovations, 3(03):226-239.
- Berek JS. Interval debulking of ovarian cancer—an interim measure. N Engl J Med. 1995;332:675–677
- Dewdney SB, Rimel BJ, Reinhart AJ, et al. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: Survey results from members of the Society of Gynecologic Oncologists. Gynecol Oncol. 2010;119:18–21.
- Hacker NF. State of the art of surgery in advanced epithelial ovarian cancer. Ann Oncol. 2013;24(suppl 10):x27–x32.
- Freedman OC, Dodge J, Shaw P, Oza AM, Bernadine M, et al. (2010) Diagnosis of epithelial ovarian carcinoma prior to neoadjuvant chemotherapy. Gynecol Oncol 119: 22-25.
- Bandyopadhyay A, Chakraborty J, Chowdhury AR, Bhattacharya A, Bhattachrya P, et al. (2012) Fine needle aspiration cytology of ovarian tumors with histological correlation. J Cytol 29:35-40.
- Goel S, Agarwal D, Goel N, Naim M, Khan T et al.(2011) Ultrasound Guided Fine Needle Aspiration Cytology In Ovarian Neoplasms: An Assessment Of Diagnostic Accuracy And Efficacy And Role In Clinical Management. The Internet Journal of Pathology 11
- Malmstrdm H (1997) Fine-needle aspiration cytology versus core biopsies in the evaluation of recurrent gynecologic malignancies. Gynecol Oncol 65: 69-73.
- Larsen T, Torp-Pedersen ST, Ottesen P, Bostofte E, Sehested M, et al. (1993) Abdominal ultrasound combined with histological and cytological fine needle biopsy of suspected ovarian tumors. Eur J Obstet Gynecol Reprod Biol 50: 203-209.
- Jha BM, Shah R, Patel J (2013) Effectiveness of image guided fine needle aspiration cytology in cases of deep seated lesions. Int J Med Sci Public Health 2: 439-442.
- Acharya Shoshan & Aryal Gopi (2024). The Diagnostic Accuracy of Frozen Section Compared to Permanent Section: A Single Center Study in Nepal Mediciti Hospital. Dinkum Journal of Medical Innovations, 3(08):550-556.
- Ghazala Mehedi,Veena Maheshwari, Sheerin Afzal, Hena A Ansari, and Maryem Ansari et al. (2010) Image guided fine needle aspiration cytology of ovarian tumors an assessment of diagnostic accuracy-J Cytol 27 (3):91-95
- Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. Radiographics. 2000 Sep-Oct. 20(5):1445-70.
- Timmerman D, Ameye L, Fischerova D, Epstein E, Melis GB, Guerriero S, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010 Dec 14. 341:c6839. [Medline].
- Nunes N, Ambler G, Foo X, Naftalin J, Widschwendter M, Jurkovic D. Use of IOTA simple rules for diagnosis of ovarian cancer: meta-analysis. Ultrasound Obstet Gynecol. 2014 Nov. 44 (5):503-14. [Medline].
- Woodward ER, Sleightholme HV, Considine AM, et al. Annual surveillance by CA125 and transvaginal ultrasound for ovarian cancer in both high-risk and population risk women is ineffective. BJOG. 2007 Dec. 114(12):1500-9. [Medline].
- Fleischer A. Ovarian cancer. Fleischer AC, Javitt MC, Jeffrey RB Jr, et al, eds. Clinical Gynecologic Imaging. Philadelphia, Pa: Lippincott Williams & Wilkins; 1996: 107.
- Yazbek J, Raju SK, Ben-Nagi J, et al. Effect of quality of gynaecological ultrasonography on management of patients with suspected ovarian cancer: a randomised controlled trial. Lancet Oncol. 2008 Feb. 9(2):124-31.
- Choi JI, Park SB, Han BH, Kim YH, Lee YH, Park HJ, et al. Imaging features of complex solid and multicystic ovarian lesions: proposed algorithm for differential diagnosis. Clin Imaging. 2015 Jul 17. [Medline].
- Mansour SM, Saraya S, El-Faissal Y. Semi-quantitative contrast-enhanced MR analysis of indeterminate ovarian tumours: when to say malignancy?. Br J Radiol. 2015 Sep. 88 (1053):20150099. [Medline].
- Bourne TH, Campbell S, Reynolds KM, et al. Screening for early familial ovarian cancer with transvaginal ultrasonography and colour blood flow imaging. BMJ. 1993 Apr 17. 306(6884):1025-9. [Medline]. [Full Text].
- Fleischer AC, Cullinan JA, Peery CV, et al. Early detection of ovarian carcinoma with transvaginal color Doppler ultrasonography. Am J Obstet Gynecol. 1996 Jan. 174(1 Pt 1):101-6
- Buy JN, Ghossain MA, Moss AA, et al. Cystic teratoma of the ovary: CT detection. Radiology. 1989 Jun. 171(3):697-701.
- Kitajima K, Kaji Y, Kuwata Y, et al. Magnetic resonance imaging findings of endometrioid adenocarcinoma of the ovary. Radiat Med. 2007 Aug 1. 25(7):346-54
- Okamoto Y, Tanaka YO, Tsunoda H, et al. Malignant or borderline mucinous cystic neoplasms have a larger number of loculi than mucinous cystadenoma: a retrospective study with MR. J Magn Reson Imaging. 2007 Jul. 26(1):94-9.
- Jaime P. Female Reproductive System. In: Damjanov. Ivan, Linder. James, editors. Anderson’s Pathology. Vol 2. 10th St.Louis; Mosby: 1996: 2231-309.
- Fadi W, Abdul-Karim, Alan B.P.Ng. Ovaries and Fallopian Tubes. In: Winifred Gray, editor. Diagnostic Cytopathology. New York: Churchill Livingstone; 1995: 811-819.
- Ganjei P. Fine Needle Aspiration Cytology of the Ovary. Clin Lab Med. 1995; 15(3): 705-726.
- Erica Wachtel, MD. The Cytology of Tumors of the Ovary and Fallopian Tubes. Clin Obstet & Gynecol. 1961; 4(4): 1159-1171.
- Ganjei P, MD., Dickinson B, M.D., Harrison TA, M.D., Nassiri M, M.D., and Y. Lu, Ph.D. Aspiration Cytology of Neoplastic and Non-Neoplastic Ovarian Cyst: Is It Accurate? Int J Gynecol Pathol 1996; 15: 94-101.
- Tahir Z, Yusuf NW, Ashraf M, Yusuf AW, Aziz F. Fine Needle Aspiration of Unilocular Ovarian Cysts – A Cytohistological Correlation. J Pak Med Assoc. 2004; 54: 266-269.
- F, Robina. F, Fehmeeda N, Nadeem. I and Ghazal.S. Role of Fine Needle Aspiration Cytology (FNAC) in Diagnosis of Uni-locular Ovarian Cysts – A Cytohistological Correlation. Biomedica. 2005; 21: 28-30.
- Uguz A, Ersoz , Bolat F, Gokdemir A, Vardar MA. Fine Needle Aspiration Cytology of Ovarian Lesions. Acta Cytol. 2005; 49(2): 144-8.
- Kar Tushar, Kar Asaranti, Mohapatra PC. Intra-operative cytology of ovarian tumours. J Obstet Gynecol India 2005; 55(4): 345-349.
- Shalinee Rao, Sadiya N, Leena Dennis Joseph, Rajendiran S. Role of scrape cytology in ovarian neoplasms. Journal of Cytology. 2009; 26(1): 26-29.
- Nazoora Khan, Nishat Afroz, Barina Aqil, Tamkin Khan, Ibne Ahmad. Neoplastic and nonneoplastic ovarian masses: Diagnosis on cytology. Journal of Cytology. 2009; 26(4): 129-133
- Sood T., Handa U., Mohan, H., Goel, P. Evaluation of aspiration cytology of ovarian masses with histopathological correlation. Cytopathol. 2010; 21(3):176-185.
- Jha B, Shah R, Patel J. Effectiveness of image guided fine needle aspiration cytology in cases of deep seated lesions, International Journal of Medical Science and Public Health,2013;2(2): 465-468
- Sengupta S, Mondal R, Bose K, Ray R, Jana S, Deoghoria D. Evaluation of Role of Ultra Sound Guided Fine Needle Aspiration Cytology for Diagnosis of Ovarian Lesions with Particular References to Diagnostic Pitfalls. Bangladesh Journal of Medical Science Vol. 13 No. 02 April’14
- Ray S, Gangopadhyay M, Bandyopadhyay A, Majumdar K, Chaudhury N. USG guided FNAC of ovarian mass lesions: A cyto-histopathological correlation, with emphasis on its role in pre-operative management guidelines, J Turk Ger Gynecol Assoc 2014; 15: 6-12
- Uguz A, Ersoz C, Bolat F, Gokdemir A, and Vardar MA. Fine Needle Aspiration Cytology of Ovarian Lesions, ACTA CYTOLOGICA 49(2):144-48
- Naguib R, Hemida R, Wageh A, Elkhiary M, Shabana A, et al. (2014) Accuracy of Combined Tru cut and FNAC in Preoperative Sampling of Ovarian Tumors. J Clin Exp Pathol 4: 168.
- Seema Goel, Deepti Agarwal, Narendra Goel, Mohd. Naim, Tamkin Khan, Ekrammulah. Ultrasound Guided Fine Needle Aspiration Cytology In Ovarian Neoplasms: An Assessment Of Diagnostic Accuracy And Efficacy And Role In Clinical Management. The Internet Journal of Pathology ISSN: 1528-8307.
- Jain R, Jain V, Dutta S, Awasthi S, Jain SK. Role of Intra-operative Cytology in the Diagnosis of Ovarian Neoplasm’s. Int J Sci Stud 2015;3(5):72-75.
- Nunez C, Diaz JI. Ovarian follicular cysts: Apotential source of false – positive diagnoses in ovarian cytology. Diagn Cytopathol 1992;8:532-7.
- Vijayakumar A. The diagnostic utility of intraoperative cytology in the management of ovarian tumours. J Clin Diagn Res 2013;7:1047-50
- Shahid M, Zaheer S, Mubeen A, Rahman K, Sherwani RK. The role of intraoperative cytology in the diagnostic evaluation of ovarian neoplasms. Acta Cytol 2012;56:467-73.
- Khunamornpong S, Siriaunkgul S. Scrape cytology of the ovaries: Potential role in intraoperative consultation of ovarian lesions. Diagn Cytopathol 2003;28:250-7.
- Wojcik EM, Selvaggi SM. Fine needle aspiration cytology of cystic ovarian lesions. Diagn Cytopathol 1994; 11: 9-14
Publication History
Submitted: April 05, 2025
Accepted: April 20, 2025
Published: May 31, 2025
Identification
D-0423
DOI
https://doi.org/10.71017/djmi.4.5.d-0423
Citation
Sharif Masuma Ismat, Chowdhury Shamima Sultana, Maksuda Begum & Latifa Akhter (2025). Efficacy of FNAC in the Diagnosis of Advanced Ovarian Malignancy. Dinkum Journal of Medical Innovations, 4(05):230-244.
Copyright
© 2025 The Author(s).


