Publication History
Submitted: March 09, 2025
Accepted: March 28, 2025
Published: March 31, 2025
Identification
D-0413
DOI
https://doi.org/10.71017/djmi.4.3.d-0413
Citation
Md. Zahirul Islam, Md. Zakir Hossain, Md. Fazle Rabby, Kuldeep Sharma, Hasan Rabbi & Md. Shakeel Ahmed (2025). Detection of Causative Agents of Acute Upper Respiratory Tract Infection by Film Array Respiratory Panel at Point of Care in a Tertiary Care Hospital . Dinkum Journal of Medical Innovations, 4(03):69-80.
Copyright
© 2025 The Author(s).
69-80
Detection of Causative Agents of Acute Upper Respiratory Tract Infection by Film Array Respiratory Panel at Point of Care in a Tertiary Care HospitalOriginal Article
Md. Zahirul Islam 1*, Md. Zakir Hossain 2, Md. Fazle Rabby 3, Kuldeep Sharma 4, Hasan Rabbi 5, Md. Shakeel Ahmed 6
- Institute for Developing Science and Health Initiatives (ideSHi), Dhaka, Bangladesh.
- Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh
- Institute for Developing Science and Health Initiatives (ideSHi), Dhaka, Bangladesh.
- Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh.
- Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh.
- Bangladesh Institute of Tropical and Infectious Diseases (BITID), Chattogram, Bangladesh.
* Correspondence: zahirul@ideshi.org
Abstract: Acute respiratory tract infections are the most prevalent illnesses in individuals of all age groups and are a significant contributor to hospitalization, morbidity, and mortality. In cases of severe infections, it is vital to quickly diagnose these infections for effective management. This study rapidly detected the causative organism of acute upper respiratory tract infection using the Film Array Respiratory Panel 2.1 plus (FARP). It was a cross-sectional study, where N=471 nasopharyngeal swab specimens were collected from suspected patients with acute upper respiratory tract infections attending the outpatient department (OPD) of the Bangladesh Institute of Tropical and Infectious Diseases (BITID). The specimens were tested with the FARP 2.1 plus, an automated multiplex PCR assay that detects 23 targets, including 19 viruses and 4 bacteria respectively. A total of 471 samples were tested by FARP 2.1 plus, and we found a notable 71% (333/471) were positive for either single (60%) or multiple (11%) pathogens. Influenza B virus (22.9%) was the most prevalent, followed by influenza A (20.2%), Human Rhino/Entero (11.3%), respiratory syncytial virus (8.1%), and SARS-COV-2 (5.2%). Influenza virus (Flu) and SARS-CoV-2 had a significant impact in single infection (P values <0.0001 and 0.0397), where Adeno virus and Bordetella pertussis had a significant impact in coinfection (P values <0.0001 and 0.001). Among the co-infections, SARS-CoV-2 and Influenza B virus (12%) were the most common, where 42% of the time was Human Rhino/Entero. The prevalence of organisms differed by age, and influenza viruses (A or B) were most common in all age groups, as in Bangladesh, the influenza virus is mostly involved in respiratory tract infections. Film Array Respiratory Panel 2.1 Plus allows the rapid simultaneous detection of a wide number of respiratory organisms, with limited hands-on time in patients with acute upper respiratory tract infection.
Keywords: acute respiratory tract infections, film array respiratory panel, real-time rt-PCR
- INTRODUCTION
Acute respiratory tract infections (ARTIs) are prevalent ailments that affect individuals of all age groups and contribute significantly to hospitalization rates and mortality. These infections have a global burden, accounting for approximately 4.0 million deaths and 454.6 million cases worldwide [1]. In 2019, ARTIs ranked as the third-leading cause of death on a global scale [2]. While various pathogens, including viruses and bacteria, can cause respiratory tract infections, it is noteworthy that the majority (~80%) of these infections are viral in nature [3]. In the general population, influenza A and B viruses, rhinoviruses, coronaviruses, respiratory syncytial viruses (RSV), adenoviruses, and parainfluenza viruses are the most commonly circulating viruses associated with ARTIs [4,5]. In addition to viruses, atypical microorganisms play a significant role in causing respiratory tract infections (RTIs) in children. One of the most common atypical microorganisms is Mycoplasma pneumoniae (M. pneumoniae), which accounts for 10-40% of children hospitalized with community-acquired pneumonia [6, 7]. Early detection of this pathogen is crucial for selecting the appropriate medication. However, diagnosing and managing patients with acute respiratory tract infections (ARTIs) can be challenging because the clinical symptoms are very similar, regardless of the causative agents. This poses a difficulty for physicians in determining the most effective treatment course, as decisions are solely based on clinical presentation. This reliance on clinical judgment can potentially result in the overuse or misuse of medications. The emergence of drug-resistant bacteria and the lack of clinical evidence pose significant global challenges. It is crucial to have data on the causative agents, improved diagnostic techniques, and the spectrum of drug sensitivity/resistance in order to effectively prevent and treat acute respiratory tract infections (ARTIs). Without this information, there is a risk of irrational and inappropriate use of antimicrobials, which contributes to the rise of multi-drug-resistant bacteria [8, 9, 10]. Accurate identification of pathogens enables clinicians to assess the necessity of further diagnostic testing, as well as the need for antibacterial or antiviral therapy. This information also aids in making decisions regarding hospitalization and implementing infection control measures [11, 12]. Currently, commonly used diagnostic methods for detecting viral or bacterial respiratory pathogens include culture and immunological techniques [13]. However, although cell culture is considered the gold standard for virus detection, it can yield inaccurate results during the early stages of the disease and is a time-consuming and laborious process [14]. Serological tests have the advantage of being faster, but they may have a low level of accuracy in terms of sensitivity and specificity [15, 16]. On the other hand, multiplex real-time reverse transcriptase PCR (RT-PCR) has been proven to be more sensitive compared to standard respiratory virus culture, bacterial culture, and antigen detection methods [17, 18]. This particular test allows for the amplification of multiple viruses or bacteria in a single reaction, making it easier to identify the most common causative agents of ARTIs [19]. However, this molecular test is technically complex and requires separate spaces. For instance, different locations are needed for sample preparation, reagent formulation, reaction setup, and amplification in order to minimize the risk of contamination and subsequent false positive results [20, 21]. Hence, there is a pressing demand for faster, more responsive, and user-friendly tests that can detect multiple respiratory pathogens simultaneously. To address this need, Film Array multiplex PCR (developed by Bio Fire Diagnostics, based in Utah, USA, and owned by bioMérieux) has been adopted by numerous laboratories globally. This molecular system comprises automated extraction of nucleic acid, an initial step of reverse transcription, and multiplex nested PCR, followed by an analysis of the melting curve [22]. The Film Array Respiratory Panel (FARP) is both approved by the FDA and CE IVD-marked. The current version of Film Array RP 2.1 plus can detect 19 viral and 4 atypical respiratory organisms respectively. The organisms detected by FARP 2.1 plus includes adenovirus, coronavirus 229E (CoV-229E), coronavirus HKU1 (CoV-HKU1), coronavirus NL63 (CoV-NL63), coronavirus OC43 (CoV-OC43), human metapneumovirus (hMPV), human rhinovirus/enterovirus (HRV/EV), influenza A (Flu A), , influenza B (Flu B), parainfluenza virus 1 (Para1), parainfluenza virus 2 (Para2), parainfluenza virus 3 (Para3), parainfluenza virus 4 (Para4), respiratory syncytial virus (RSV), Middle East Respiratory Syndrome (MERS-Co V), Bordetella pertussis (detection of ptx P), Bordetella Para pertussis (detection of IS1001), Chlamydia pneumonia and Mycoplasma pneumonia [23]. Because the human rhinoviruses and enteroviruses share genetic similarities, a positive outcome using PCR primers for these viruses was recorded as Rhino/Entero. In addition, the Flu A viruses could be further categorized as influenza A H1 (Flu A H1), influenza A H1-2009 (Flu A H1-2009), and influenza A H3 (Flu A H3) if they were present. The testing process is conducted within a closed system, requiring 5 minutes of direct involvement and 45 minutes of instrumentation time. There is limited data available on the application of the Film Array respiratory panel in detecting ARTI pathogens in symptomatic patients in Bangladesh. Therefore, this study aims to rapidly detect the causative organism of acute upper respiratory tract infection by Film Array Respiratory Panel 2.1 plus (FARP) in outpatients visiting BITID with suspected respiratory tract infections.
- MATERIALS & METHOD
In this cross-sectional study, we collected nasopharyngeal swab specimens from N=471 individuals suspected of having acute respiratory tract infections. These patients visited the outpatient department (OPD) of the Bangladesh Institute of Tropical and Infectious Diseases (BITID). On the sampling day, we obtained written consent from the patients. In the case of child individuals, we got written consent from their parents. The Institutional Review Board (IRB) of the BITID approved the ethical permission for this study. We included an individual of any age suspected of having an acute respiratory tract infection characterized by the following symptoms: cough, fever, rhinitis, sore throat, nasal congestion, sneezing, headache, wheezing, throat discomfort, muscle ache, chest tightness, and shortness of breath within 7 days of appearance. Nasopharyngeal Swab (NPS) was collected according to standard procedure. To collect the NPS, a sterile swab made of cotton fibers was inserted into the nostrils, reaching approximately 1 to 1.5 cm, and rotated against the anterior nasal mucosa for 3 seconds. Then, the NPS sample was immediately placed in viral transport media (VTM). Specimens should be tested as early as possible. If there is a need for storage of the specimens, we follow the manufacturer’s instructions [23]. Respiratory samples were examined for pathogens using the Film Array respiratory panel 2.1 plus, capable of detecting 19 viruses and 4 atypical bacteria. The NPS specimen’s 300 μl of viral transport media (VTM) was mixed with 500 μl of sample buffer and injected into the pouch’s sample port as per the manufacturer’s instructions. All suspected specimens were handled in a biosafety cabinet with full personal protective equipment. The pouch’s barcode was then scanned using a barcode scanner. Once the pouch was placed in the machine, the automated process began immediately. The Film Array RP2.1 plus panel test involves automated extraction of nucleic acid, reverse transcription, and the first stage of a multiplexed PCR, followed by an individual nested second-stage real-time PCR on a microarray chip. Results are analyzed in approximately 45 minutes per run, with each target analyzed in triplicate. The Film Array Torch Software (version 3.1.317.0) from BioFire/BioMérieux performs automated result analysis using melting curve data, with each target in a valid run reported as either ‘Detected’ or ‘Not Detected. ‘ The Film Array RP2.1 plus incorporates two internal controls: one for RNA processing and controls for each step within the pouch. In the event that either internal control fails, the software will automatically generate an ‘Invalid’ result for all panel analytes. The data analysis was conducted using the Statistical Package for Social Science (SPSS), version 23.0 from IBM Corporation. Continuous variables were expressed as means ± SD, while categorical variables were represented by frequency and percentages. The comparison of categorical variables was conducted using the Chi-square test. Fisher’s exact test was done to compare pathogen frequencies. For statistical significance, P < 0.05 was considered.
- RESULTS & DISCUSSION
A total of 471 samples were examined using the Film array Respiratory Panel 2.1 plus. Among these samples, 276 (59%) were male, and 195 (41%) were female. The average age was 29.8±15.8, with a median age of 28. It is worth noting that the largest proportion of participants fell within the 11-30 age group, accounting for 49% of the total. This was followed by the 31-50 age group, which made up 30% of the participants. There were also extremes in age, with 9% of participants being 10 years or younger and 12% being over 50 years old. As for clinical symptoms, a significant number of participants displayed symptoms, with cough being the most prevalent (86%), closely followed by fever (82%) Table 01.
Table 01: Demographic and Clinical presentation of ARTI cases
| Variable | Number (%) |
| Sex | |
| Male | 276 (59%) |
| Female | 195 (41%) |
| Age, Years | |
| Mean | 29.6± 15.8 |
| Median | 28 |
| Age Groups | |
| ≤10 years | 43 (9%) |
| 11-30 years | 231 (49%) |
| 31-50 years | 142 (30%) |
| >50 years | 55 (12%) |
| Clinical features | |
| Symptomatic | |
| Fever | 386 (82%) |
| Cough | 407 (86%) |
| Sore Throat | 85 (18%) |
| Runny Nose | 49 (10%) |
| Shortness of Breath | 35 (7%) |
| Headache | 35 (7%) |
| Others | 24 (5%) |
The positivity rate of ARTI detected by Bio fire Film Array Respiratory panel 2.1 plus. Out of the 471 individuals included in the sample, it was found that a significant 71% (333 out of 471) tested positive for the condition being investigated. When examining the distribution based on gender, it was observed that 61% of males and 39% of females tested positive for the condition (p>0.05). Furthermore, individuals between the ages of 11-30 had the highest rate of positivity at 51%, followed by those in the 31-50 age group with a rate of 29%. The lowest rate of positivity was observed in individuals ≤10 years old at 9%, while individuals older than 50 had a rate of 11%, p>0.05 (Table 02).
Table 02: Positivity rate of ARTI cases using Bio fire Film Array Respiratory panel 2.1 plus
| All Samples tested (n=471) | Number (%) |
| All Positive | 333 (71) |
| Sex | |
| Male | 202 (61) |
| Female | 131 (39) |
| Age Group | |
| ≤10 | 29 (9) |
| 11-30 | 170 (51) |
| 31-50 | 97 (29) |
| >50 | 37 (11) |
A total of 71% of the samples were identified by the Film Array test. Out of 471 samples, 60% (283/471) detected a single pathogen, 11% (50/471) detected multiple pathogens, and 29% (138/471) did not have any pathogen present. The most predominant pathogen detected overall was the influenza virus accounting for over 43% of all tested specimens with which influenza B (Flu B), accounting for 22.9% (108/471) of cases, followed by influenza A (Flu A) at 20.2% (95/471), Human Rhino/ Entero at 11.3% (53/471), and respiratory syncytial virus (RSV) at 8.1% (38/471) respectively (Figure 1). Among the Influenza A strains, H3 and H1-2009 were detected at rates of 10% and 8%, respectively. We found 2.2% of Influenza A with no subtype detected.
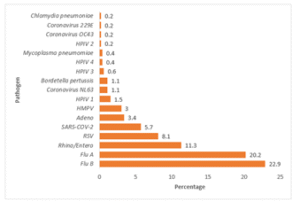
Figure 01: Positive spectrum of Respiratory pathogen detected by Film Array Respiratory panel 2.1 plus
The frequency of single infections of Flu and SARS-CoV-2 was higher than that of coinfection cases (P values <0.0001 and 0.0397). Single infection cases for Rhino/Entero and RSV were higher than those of coinfection, but the difference did not generate a significant P value. In the case of other viruses and bacteria, we observed that there were no significant differences between single infection and coinfection cases except Adeno virus and B. pertussis show higher proportion of coinfection (P values <0.0001 and 0.001) respectively (Figure 02).
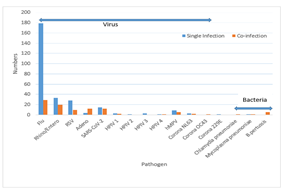
Figure 02: Comparison of viral and bacterial frequency in single infection and coinfection
A total of 50 different types of mixed organisms were identified, with 27 different combinations. Out of the 471 specimens, 11% (50/471) tested positive for more than one analyte. The most common combination was the presence of both SARS-CoV-2 and Influenza B, which was found in 12% (6/50) of the cases. This was followed by a combination of Adenovirus and Influenza B, which accounted for 10% (5/50) of the cases. Other common combinations included Human Rhino/Entero and Influenza B (8%, 4/50), as well as Human Rhino/Entero and Adenovirus (8%, 4/50) (Table 3). Among the mixed organism-positive patients, the majority were observed to have Human Rhino/Entero (42%, 21/50), Influenza B (36%, 18/50), SARS-CoV-2 (24%, 12/50), Adenovirus (24%, 12/50), and RSV (18%, 9/50) (Figure 03).
Table 03: Distribution of mixed-pathogens combination in NPS specimens
| Analyte 1 | Analyte 2 | Analyte 3 | Number |
| SARS-CoV-2 | Influenza B | 6 | |
| Adenovirus | Influenza B | 5 | |
| Human Rhinovirus/Enterovirus | Influenza B | 4 | |
| Human Rhinovirus/Enterovirus | Adenovirus | 4 | |
| Human Rhinovirus/Enterovirus | Influenza A H1-2009 | 3 | |
| Human Rhinovirus/Enterovirus | Influenza A H3 | 3 | |
| Respiratory syncytial virus | Human Rhinovirus/Enterovirus | 2 | |
| Respiratory syncytial virus | Influenza B | 2 | |
| Human Rhinovirus/Enterovirus | Bordetella pertussis | 2 | |
| SARS-COV-2 | Influenza A H3 | 2 | |
| Respiratory syncytial virus | Influenza A (no subtype detected) | 1 | |
| Respiratory syncytial virus | Mycoplasma pneumoniae | 1 | |
| Respiratory syncytial virus | Coronavirus NL63 | 1 | |
| Respiratory syncytial virus | Adenovirus | 1 | |
| Respiratory syncytial virus | Human Metapneumovirus | 1 | |
| Human Rhinovirus/Enterovirus | Human Metapneumovirus | 1 | |
| Human Rhinovirus/Enterovirus | SARS-CoV-2 | 1 | |
| Human Rhinovirus/Enterovirus | Human Metapneumovirus | 1 | |
| SARS-COV-2 | Parainfluenza Virus 4 | Bordetella pertussis | 1 |
| SARS-COV-2 | Parainfluenza Virus 1 | 1 | |
| SARS-COV-2 | Influenza A H1-2009 | 1 | |
| Adenovirus | Coronavirus NL63 | 1 | |
| Adenovirus | Chlamydia pneumoniae | 1 | |
| Influenza A H3 | Human Metapneumovirus | Bordetella pertussis | 1 |
| Influenza A H3 | Human Metapneumovirus | 1 | |
| Influenza B | Parainfluenza Virus 1 | 1 | |
| Coronavirus OC43 | Parainfluenza Virus 1 | Bordetella pertussis | 1 |
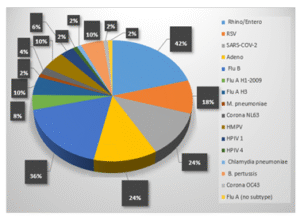
Figure 03: The percentage of mixed-pathogen positive specimens positive for the specific pathogen
In all age groups, Influenza B (IFV B) and Influenza A (IFV A) are the most common viral pathogens. Both IFV A and IFV B contribute significantly to the overall number of influenza cases. The third highest viral pathogen found in all age groups is Human Rhino/Enterovirus. Other viral pathogens detected did not show significant prevalence across different age groups. Additionally, only one positive case of viral pathogens for Coronavirus 229E, Coronavirus OC43, and HPIV 2 was found in the 31-50 years and 11-30 years age groups. Among bacterial pathogens, Bordetella pertussis and Mycoplasma pneumoniae were observed in the 11-30 years age group. Furthermore, there was only one patient who tested positive for C. pneumoniae during the study, and this patient belonged to the > 50year age group. No positive results were found for Coronavirus HKU1, MERS, and Bordetella Para pertussis during the study period (Table 04). Acute respiratory tract infection is a significant health issue that has a profound impact on patients and society. It is particularly prevalent in children and certain individuals with compromised immune systems [24, 25]. The use of molecular diagnosis, specifically the multiplex PCR assay, has proven to be a more effective tool compared to routine diagnostic tests. This study rapidly detected respiratory organisms using Film Array RP2.1 plus and to provide insights into their prevalence. A total of 471 patients were included in the study, with 276 being male and 195 females. There was no significant difference in the positive rate between genders. However, there were noticeable differences observed among various age categories. We found that the prevalence of pathogens in the age groups of 11 to 30 years and 31 to 50 years was higher compared to other groups. Various factors, such as lifestyle choices, social interactions, the development of the immune system, and exposure to crowded environments like schools and workplaces, may contribute to the increased vulnerability of respiratory pathogens among adults. However, a study revealed that the rates of pathogen positivity were notably higher in infants (0-6 months), toddlers (7 months – 2 years), and children (3-6 years) compared to other groups [26]. Another study also identified the highest rate of positivity at 6 years of age [27]. We analyzed 471 suspected samples of acute respiratory tract infections using the Bio fire Film Array Respiratory Panel 2.1 plus. Our findings showed that 71% of the samples tested positive for either a single pathogen (60%) or multiple pathogens (11%). These results align with previous reports from diverse countries [28, 29, 30]. Previous research suggests that the influenza virus plays a significant role in respiratory infections worldwide. Our study reveals that the influenza virus is predominant among patients with acute respiratory tract infections (ARTI), accounting for over 43% of all tested specimens. These findings align with earlier studies [31, 32, 33]. While most studies have identified influenza type A as the dominant strain [32, 34, 35], we observed a slightly higher prevalence of type B (22.9%) compared to type A (20.2%), which is consistent with a few other studies [36, 37].
Table 04: Prevalence of respiratory pathogens tested in different age groups by Bio fire Film Array Respiratory panel 2.1 plus
| Pathogen | ≤10 yrs | 11-30 yrs | 31-50 yrs | >50 yrs | ||||
| Virus | Pos | Prevalence
n=43 |
Pos | Prevalence
n=231 |
Pos | Prevalence
n=142 |
Pos | Prevalence
n=55 |
| IFV B | 11 | 25.6% | 58 | 25.1% | 31 | 21.8% | 8 | 14.5% |
| IFV A | 8 | 18.6% | 45 | 19.5% | 26 | 18.3% | 11 | 20% |
| Flu A H1 | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Flu A H3 | 6 | 13.9% | 26 | 11.3% | 11 | 7.7% | 5 | 9% |
| Flu A H1-2009 | 2 | 4.6% | 18 | 7.8% | 13 | 9.2% | 5 | 9% |
| Flu A (no subtype) | 0 | 0% | 3 | 1.3% | 2 | 1.4% | 0 | 0% |
| Human Rhino/Entero | 5 | 11.6% | 27 | 11.7% | 14 | 9.9% | 7 | 12.7% |
| Adeno | 2 | 4.7% | 5 | 2.2% | 6 | 4.2% | 3 | 5.5% |
| RSV | 2 | 4.7% | 21 | 9% | 10 | 7% | 5 | 9% |
| Coronavirus 229E | 0 | 0% | 0 | 0% | 1 | 0.7% | 0 | 0% |
| Coronavirus HKU1 | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Coronavirus NL63 | 0 | 0% | 0 | 0% | 3 | 2.1% | 2 | 3.6% |
| Coronavirus OC43 | 0 | 0% | 1 | 0.4% | 0 | 0% | 0 | 0% |
| SARS-CoV-2 | 2 | 4.6% | 14 | 6% | 6 | 4.2% | 5 | 9% |
| HPIV 1 | 1 | 2.3% | 3 | 1.3% | 2 | 1.4% | 1 | 1.8% |
| HPIV 2 | 0 | 0% | 1 | 0.4% | 0 | 0% | 0 | 0% |
| HPIV 3 | 1 | 2.3% | 0 | 0% | 2 | 1.4% | 0 | 0% |
| HPIV 4 | 0 | 0% | 1 | 0.4% | 1 | 0.7% | 0 | 0% |
| HMPV | 0 | 0% | 8 | 3.5% | 1 | 0.7% | 2 | 3.6% |
| MERS | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Bacteria | ||||||||
| Bordetella Para pertussis | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Bordetella pertussis | 0 | 0% | 5 | 2.2% | 0 | 0% | 0 | 0% |
| Mycoplasma pneumoniae | 0 | 0% | 2 | 0.9% | 0 | 0% | 0 | 0% |
| Chlamydia pneumoniae | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 1.8% |
| Total | 29 | 9% | 170 | 51% | 97 | 29% | 37 | 11% |
| One or more pathogens | ||||||||
Other respiratory viruses, such as Human Rhino/Entero (11.3%), RSV (8.1%), and SARS-CoV-2 (5.7%), co-circulated with influenza viruses but at lower rates. It is increasingly recognized that simultaneous infection with multiple pathogens is common and can significantly impact disease manifestation. We identified that 50 patients had more than two microorganisms, with SARS-CoV-2 and Influenza B virus being the most prevalent. Previous studies have shown that influenza A virus was more commonly found as a co-infection with SARS-CoV-2 [38]. However, in our research, Influenza B virus was reported as a co-infection pathogen in 6 cases, compared to 3 cases with influenza A virus. Through multiplex respiratory PCR, our study detected co-infections in 11% of cases, with Human Rhino/Entero (42%) being the most frequently observed. Other studies have reported slightly lower rates of multiple-positive specimens, around 10% and 8.7%, respectively [39, 40]. The clinical significance of multi-pathogen infections, especially those involving the Rhino/Entero combination, including disease severity and hospitalization time, remains uncertain. A previous study suggested that viral shedding from an earlier Rhino/Entero infection may be the cause of the dual-positive results with Rhino/Entero and RSV [41]. We observed the prevalence of respiratory pathogens among different age groups. In all age groups, both IFV A and IFV B were found to be contributing factors. In Bangladesh, the influenza virus is primarily associated with respiratory tract infections. A similar finding was reported in a previous study [42]. However, this study has certain limitations. Firstly, it was conducted at a single center and may not be representative of the entire population in Chattogram. Secondly, due to limited resources, we were unable to obtain data from a more suitable assay to assess the sensitivity and specificity of Film Array RP2.1 plus.
- CONCLUSIONS
The study comprehensively evaluated the etiological profile of acute upper respiratory tract infections (ARTIs) using the Film Array Respiratory Panel 2.1 plus (FARP) in a tertiary care setting in Bangladesh. The results underscore the significance of rapid molecular diagnostics in identifying causative agents, optimizing patient management, and curbing inappropriate antibiotic use. Out of 471 nasopharyngeal swab samples tested, an impressive 71% yielded positive results, highlighting the efficiency of FARP in detecting respiratory pathogens with a high level of accuracy and speed. The study found that single infections were more common than co-infections, with 60% of patients harboring a single pathogen and 11% presenting with multiple pathogens. Among the detected organisms, influenza viruses emerged as the most predominant pathogens, collectively accounting for over 43% of all positive cases. Notably, influenza B virus (22.9%) surpassed influenza A (20.2%) in prevalence, a finding that aligns with select international studies but contrasts with the more common global trend of influenza A predominance. Subtyping revealed that influenza A H3 (10%) and influenza A H1-2009 (8%) were the most frequently observed strains. These results emphasize the critical role of influenza viruses as a major driver of ARTIs in Bangladesh. The distribution of infections varied across age groups, with individuals aged 11–30 years (51%) and 31–50 years (29%) showing higher prevalence rates. This age-related trend may be attributed to lifestyle factors, increased social interactions, and occupational exposure. Conversely, younger children and older adults showed comparatively lower infection rates in this cohort, though global literature often emphasizes heightened vulnerability in pediatric and geriatric populations. Gender, however, did not show a significant influence on infection rates, with both males and females exhibiting comparable positivity. The findings collectively highlight the utility of Biofire FilmArray respiratory panel assay as a rapid, reliable, and comprehensive diagnostic tool capable of identifying 23 respiratory organisms within 45 minutes. Its use enhances clinical decision-making by enabling timely and targeted therapy, thereby reducing unnecessary antibiotic prescriptions and minimizing the risk of antimicrobial resistance. Furthermore, the ability to detect both viral and atypical bacterial pathogens positions this technology as a cornerstone for strengthening respiratory infection surveillance and guiding public health strategies. In conclusion, influenza viruses, particularly type B, remain the dominant cause of ARTIs in Bangladesh, with notable contributions from Rhino/Entero, RSV, and SARS-CoV-2. The detection of co-infections, especially involving SARS-CoV-2 and influenza viruses, underscores the evolving epidemiology of respiratory pathogens in the post-pandemic era. Implementation of molecular diagnostic platforms such as the Film Array RP2.1 plus in clinical practice can significantly improve patient outcomes by ensuring rapid diagnosis, guiding rational antimicrobial use, and shaping effective infection control measures. Future multicenter studies with larger populations are warranted to generalize these findings and to explore the clinical implications of coinfections in greater depth.
REFERENCES
- Litwin CM, Bosley JG. Seasonality and prevalence of respiratory pathogens detected by multiplex PCR at a tertiary care medical center. Arch Virol. 2014 Jan; 159(1):65-72.
- Global burden of chronic respiratory diseases and risk factors, 1990–2019: an update from the Global Burden of Disease Study 2019
- Noor Alam Ansari, Sanjay Prasad Sah, Susanta Kumar Paul, Pratik Wagley, Shamim Ahmed & Mohammed Atiqur Rahman (2025). Assessment of Vitamin D 25(OH)D Status in Patients with Tuberculous Pleural Effusion in a Tertiary Care Hospital: A Cross-Sectional Study. Dinkum Journal of Medical Innovations, 4(01):15-28.
- Kenmoe S, Bigna JJ, Well EA, Simo FBN, Penlap VB, Vabret A, Njouom R. Prevalence of human respiratory syncytial virus infection in people with acute respiratory tract infections in Africa: A systematic review and meta-analysis. Influenza Other Respir Viruses. 2018 Nov;12(6):793-803.
- Sulav Regmi, Achyut Neupane & Deepika Joshi (2024). Effect of Stubble Burning on Respiratory Function and Quality of Life in a Rural Community (Punjab, India) – A Prospective Observational Study. Dinkum Journal of Medical Innovations, 3(11):761-783.
- Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015; 372(9): 835–45.
- Liu WK, Liu Q, Chen DH, Liang HX, Chen XK, Chen MX, Qiu SY, Yang ZY, Zhou R. Epidemiology of acute respiratory infections in children in Guangzhou: a three-year study.
- Rakesh Kumar Mahato, Sagar Pokharel & Avinash Sahani (2024). Knowledge & Practice Regarding Neonatal Resuscitation among Health Care Providers in Tertiary Care Hospitals of Nepal. Dinkum Journal of Medical Innovations, 3(03):257-270.
- Cillo´niz C, Dominedò C, Torres A. Multidrug resistant gram-negative bacteria in community-acquired pneumonia. Annual Update in Intensive Care and Emergency Medicine 2019. 2019; p. 459–475.
- Song JH, Chung DR. Respiratory infections due to drug-resistant bacteria. Infectious Disease Clinics. 2010; 24(3):639–653. PMID: 20674796
- Barenfanger J, Drake C, Leon N, Mueller T, Troutt T (2000) Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J Clin Microbiol 38: 2824–2828.
- Mahony JB, Blackhouse G, Babwah J, Smieja M, Buracond S, Chong S, Ciccotelli W, O’Shea T, Alnakhli D, Griffiths-Turner M, Goeree R. Cost analysis of multiplex PCR testing for diagnosing respiratory virus infections. J Clin Microbiol. 2009 Sep; 47(9):2812-7.
- Tsuchiya LR, Costa LM, Raboni SM, Nogueira MB, Pereira LA, Rotta I, Takahashi GR, Coelho M, Siqueira MM (2005) Viral respiratory infection in Curitiba, Southern Brazil. J Infect 51:401–407
- Leland DS, Ginocchio CC. Role of cell culture for virus detection in the age of technology. Clin Microbiol Rev. 2007; 20: 49–78.
- Zumla A, Al-Tawfiq JA, Enne VI, Kidd M, Drosten C, Breuer J, et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections—needs, advances, and future prospects. Lancet Infect Dis. 2014; 14: 1123.
- Liu L, Liu W, Zheng Y, Jiang X, Kou G, Ding J, et al. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect. 2020; 22: 206.
- Liao RS, Tomalty LL, Majury A, Zoutman DE (2009) Comparison of viral isolation and multiplex real-time reverse transcription-PCR for confirmation of respiratory syncytial virus and influenza virus detection by antigen immunoassays. J Clin Microbiol 47:527–532
- Nolte FS, Marshall DJ, Rasberry C, Schievelbein S, Banks GG, Storch GA, Arens MQ, Buller RS, Prudent JR (2007) MultiCodePLx system for multiplexed detection of seventeen respiratory viruses. J Clin Microbiol 45:2779–2786
- Huang HS, Tsai CL, Chang J, Hsu TC, Lin S, Lee CC. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis. Clin Microbiol Infect. 2018; 24: 1055– 1063.
- Mothershed EA, Whitney AM (2006) Nucleic acid-based methods for the detection of bacterial pathogens: present and future considerations for the clinical laboratory. Clin Chim Acta 363: 206–220.
- Yang S, Rothman RE (2004) PCR-based diagnostics for infectious diseases: uses limitations, and future applications in acute-care settings. Lancet Infect Dis 4: 337–348
- Poritz MA, Blaschke AJ, Byington CL, Meyers L, Nilsson K, Jones DE, Thatcher SA, Robbins T, Lingenfelter B, Amiott E, et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection.
- BioFire® Respiratory Panel 2.1-EZ (RP2.1-EZ)
- Shorr, A. F. et al. Viruses are prevalent in non-ventilated hospital-acquired pneumonia. Respir. Med. 122, 76–80 (2017).
- Nguyen, C. et al. viral respiratory infections of adults in the intensive care unit. J. Intensive Care Med. 31, 427–441 (2016)
- Zhang, J., Yang, T., Zou, M. et al. The epidemiological features of respiratory tract infection using the multiplex panel’s detection during the COVID-19 pandemic in Shandong province, China. Sci Rep 13, 6319 (2023).
- Christy S. Stover, Christine M. Litwin, “The Epidemiology of Upper Respiratory Infections at a Tertiary Care Center: Prevalence, Seasonality, and Clinical Symptoms”, Journal of Respiratory Medicine, vol. 2014, Article ID 469393, 8 pages, 2014
- Dia N, Sarr FD, Thiam D, Sarr TF, Espie´ E, OmarBa I, et al. Influenza-like illnesses in Senegal: not only focus on influenza viruses. PloS One. 2014; 9.
- Adam K, Pangesti KNA, Setiawaty V. Multiple Viral Infection Detected from Influenza-Like Illness Cases in Indonesia. BioMed Res Int. 2017;2017.
- Al-Romaihi HE, Smatti MK, Al-Khatib HA, Coyle PV, Ganesan N, Nadeem S, et al. Molecular epidemiology of influenza, RSV, and other respiratory infections among children in Qatar: A six years report (2012–2017). Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020; 95: 133–141
- Dangi T, Jain B, Singh AK, Singh JV, Kumar R, Dwivedi M, et al. Molecular characterization of circulating pandemic strains of influenza A virus during 2012 to 2013 in Lucknow (India). J Med Virol. 2014; 86: 2134–2141. https://doi.org/10.1002/jmv.23946 PMID: 24777528
- Ye C, Zhu W, Yu J, Li Z, Zhang Y, Wang Y, et al. Understanding the complex seasonality of seasonal influenza A and B virus transmission: Evidence from six years of surveillance data in Shanghai, China. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2019; 81: 57–65.
- Machablishvili A, Chakhunashvili G, Zakhashvili K, Karseladze I, Tarkhan-Mouravi O, Gavashelidze M, et al. Overview of three influenza seasons in Georgia, 2014–2017. PloS One. 2018; 13.
- Caini S, El-Guerche Se´blain C, Ciblak MA, Paget J. Epidemiology of seasonal influenza in the Middle East and North Africa regions, 2010–2016: Circulating influenza A and B viruses and spatial timing of epidemics. Influenza Other Respir Viruses. 2018; 12: 344–352.
- Seleka M, Treurnicht FK, Tempia S, Hellferscee O, Mtshali S, Cohen AL, et al. Epidemiology of influenza B/Yamagata and B/Victoria lineages in South Africa, 2005–2014. PloS One. 2017; 12.
- Korsun NS, Angelova SG, Trifonova IT, Georgieva IL, Tzotcheva IS, Mileva SD, et al. Predominance of influenza B/Yamagata lineage viruses in Bulgaria during the 2017/2018 season. Epidemiol Infect. 2019; 147: 1–8.
- Jennings Z, Carter I, McPhie K, Kok J, Dwyer DE. Increased prevalence of influenza B/Victoria lineage viruses during early stages of the 2015 influenza season in New South Wales, Australia: implications for vaccination and planning. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2015; 20.
- Sominina A, Danilenko D, Komissarov A, Karpova L, Pisareva M, Fadeev A, et al. Resurgence of Influenza Circulation in the Russian Federation during the Delta and Omicron COVID-19 Era. Viruses. 2022; 14.
- Mahony JB (2008) Detection of respiratory viruses by molecular methods. Clin Microbiol Rev 21:716–747
- Olofsson S, Brittain-Long R, Andersson LM, Westin J, Lindh M (2011) PCR for detection of respiratory viruses: seasonal variations of virus infections. Expert Rev Anti Infect Ther 9:615 626
- Brittain-Long R, Westin J, Olofsson S, Lindh M, Andersson LM. Prospective evaluation of a novel multiplex real-time PCR assay for detection of fifteen respiratory pathogens-duration of symptoms significantly affects detection rate. J Clin Virol. 2010;47(3):263–7
- Zaman RU, Alamgir ASM, Rahman M, Azziz-Baumgartner E, Gurley ES, Sharker MAY, et al. (2009) Influenza in Outpatient ILI Case-Patients in National Hospital-Based Surveillance, Bangladesh, 2007–2008. PLoS ONE 4(12): e8452. Abro, S. U., Saleem, Q., Begum, A., Azhar, S., Naseer, A., & Qureshi, A. A. (2020). Association of BMI (Body Mass Index) to haemoglobin and red blood cell indices among adolescents. The Professional Medical Journal, 27(10), 2210-2215.
Publication History
Submitted: March 09, 2025
Accepted: March 28, 2025
Published: March 31, 2025
Identification
D-0413
DOI
https://doi.org/10.71017/djmi.4.3.d-0413
Citation
Md. Zahirul Islam, Md. Zakir Hossain, Md. Fazle Rabby, Kuldeep Sharma, Hasan Rabbi & Md. Shakeel Ahmed (2025). Detection of Causative Agents of Acute Upper Respiratory Tract Infection by Film Array Respiratory Panel at Point of Care in a Tertiary Care Hospital . Dinkum Journal of Medical Innovations, 4(03):69-80.
Copyright
© 2025 The Author(s).


