Publication History
Submitted: February 19, 2025
Accepted: March 02, 2025
Published: April 30, 2025
Identification
D-0418
DOI
https://doi.org/10.71017/djmi.4.4.d-0418
Citation
Bibek Lamichhane (2025). Study of Iron Profile and Vitamin B12 in Patients with Heart Failure with Reduced Ejection Fraction. Dinkum Journal of Medical Innovations, 4(04):147-164.
Copyright
© 2025 The Author(s).
147-164
Study of Iron Profile and Vitamin B12 in Patients with Heart Failure with Reduced Ejection FractionOriginal Article
Bibek Lamichhane 1 *
- Department Of Internal Medicine, Manipal College of Medical Sciences, Pokhara, Nepal.
* Correspondence: gilmourdavidbibek@gmail.com
Abstract: Iron Deficiency and Vitamin B12 deficiency are one of the commonest nutritional deficiencies worldwide, affecting large numbers of the population being more common in resource limited country like Nepal. It is of global concern that there might be association of these deficiencies with or without anemia with heart failure. This study accessed the Iron profile and Vitamin B12 level in patients with HFrEF admitted in Manipal Teaching Hospital. Serum Iron, Ferritin, and Transferrin Saturation was calculated and the prevalence of iron deficient and Vitamin B12 deficient cases with HFrEF was studied. It was a hospital based observational cross-sectional study, a total of N=57 patients who had a diagnosis of heart failure with reduced ejection fraction based on the Framingham Criteria and Echocardiography meeting all the inclusion and exclusion criteria were included. They underwent laboratory evaluation including hemoglobin concentration, serum iron, transferrin saturation percentage, serum ferritin, total iron binding capacity. Iron deficiency was defined as Serum ferritin <100 μg/l or Serum ferritin level of 100– 300 μg/l and a transferrin saturation of <20 %. Vitamin B12 deficiency was defined as level <239pg/ml. Anemic females were more than anemic males (55.9% vs 44.1%) and it was statistically significant. (p< 0.01) The prevalence of anemia was increasing with the age, highest being over 70 years of age. Iron deficient cases with anemia were 66.7%. Three fourth (75.4%) cases had transferrin saturation less than 20%. Vitamin B12 deficiency was prevalent in one fourth (28%) of cases with female preponderance and was found statistically significant (p= 0.02). Iron deficiency was prevalent in patients with heart failure and reduced ejection fraction irrespective of anemia and hemoglobin levels. In female gender, the association of HFrEF with anemia was statistically significant. Contrary to our expectation, Vitamin B12 deficiency prevalence was relatively low in our study and statistically significant association was seen in female population. Measurement of iron status could be considered during workup of heart failure patients.
Keywords: anemia, heart failure, hemoglobin, iron deficiency
- INTRODUCTION
Heart failure (HF) can be defined as the syndrome that results from structural and functional defects in myocardium resulting in impairment of ejection of blood or filling of ventricle. The most common cause that will result in HF is reduced left ventricular myocardial function [1]. Also, alterations in functions of the pericardium, myocardium, endocardium, heart valves alone or in combination also predispose to heart failure. Various pathophysiologic mechanisms leading to HF include ischemia, increased hemodynamic overload, abnormality in myocyte calcium cycling, remodeling of ventricles, inflammatory changes in myocytes and interstitial, biochemical alterations or accelerated apoptosis and mutations [1,2]. Virtually any heart disease can land into heart failure [3]. HF has been divided into distinct phenotypes based on the measurement of left ventricular ejection fraction Reduced LVEF is defined as ≤40%, i.e. those with a significant reduction in LV systolic function. This is designated as HFrEF. Patients with a LVEF between 41% and 49% have mildly reduced LV systolic function, i.e. HF mrEF. Those with symptoms and signs of HF, with evidence of structural and/or functional cardiac abnormalities and/or raised natriuretic peptides (NPs), and with an LVEF ≥50%, have HFpEF [4]. The most commonly used modality for assessment of LVEF is echocardiography [5]. The increase in blood volume in severe cases of anemia with hemoglobin level of approximately 7g/dl in iron deficient patient causes the transition from compensated high output cardiac state to a state of decompensated left ventricular function [6]. Iron deficiency (ID) is an important co-morbid condition in patients with HF [7]. ID results in impairment of functional status of heart even in the absence of anemia, whereas iron repletion therapy is beneficial regardless of the presence of anemia [8]. The iron deficiency anemia (IDA) is defined as a hemoglobin level <12 g/dL in women and <13 g/dL in men; serum ferritin level <12 ng/mL [9]. In HF, the criteria for diagnosing iron deficiency are not uniform but generally include an absolute ferritin level <100μg/l and ferritin level100–300 μg/l in combination with a transferrin saturation<20% [10]. Heart failure is a state of oxidative stress leading to inactivation of vitamin B12 metabolites causing a functional vitamin B12 deficiency state [11]. The cutoff value of vitamin B12 derived from a local reference range is required to diagnose vitamin B12 deficiency in the presence of a strong clinical suspicion [12]. Inadequate intake of vitamin B12 and other vitamins, are associated with a slight increase in HF risk [13]. Vitamin B12 deficiency in HF cases is relatively rare compared to other micronutrients deficiency, and the prognostic value of vitamin B12 deficiency is yet to be determined in these cases [14]. In the past, heart failure was described and mentioned in ancient texts of Egypt, Greece and India. Foxgloves were used as medicines by the Romans. For centuries treatment through leeches and Blood-letting were used for heart failure and some of other similar conditions. But after scientific description of nature of circulation in 1628 by William Harvey, a scientific knowledge of the disease was begun to be understood [15]. The 2021 European Society of Cardiology has quoted a definition of Heart failure as “HF is not a single pathological diagnosis, but a clinical syndrome consisting of cardinal symptoms (e.g. breathlessness, ankle swelling, and fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles, and peripheral oedema). It is due to a structural and/or functional abnormality of the heart that results in elevated intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise.” To identify the underlying cause of cardiac dysfunction is crucial in the diagnosis of HF as subsequent treatment depends on the specific pathology. HF is commonly is due to myocardial dysfunction: either systolic, diastolic, or both. However, any pathology in the valves, pericardium, endocardium, and abnormalities in the rhythm and conduction of cardiac impulse can also cause or contribute to HF [4]. This definition focuses only on HF cases who are clinically symptomatic. There can be asymptomatic phase before this symptomatic stage where there is only structural and / or functional pathology in the cardiac tissue. (e.g. systolic and / or diastolic dysfunction of left ventricle). Such pathology can be precursors of cardiac failure. However, if drugs are initiated against these initial findings of heart failures, it could halt the disease progression and may even decrease the mortality of heart failure [17,18]. HFrEF was termed classically as “systolic heart failure” while HFpEF was thought as “diastolic heart failure”. But as far as our present understandings, cases of HFrEF may also have some disorder in diastolic function, and HFpEF cases may show minimal amount of systolic dysfunction. Terms like “systolic” and “diastolic” heart failure have become obsolete because of these reasons. One important point that should be noted is left ventricular function does not correlate with severity of symptoms. There is high mortality in patients with severe symptoms, it does not necessitate that here is reduced risk of hospitalization and death in patients with mild symptoms [19,20]. In patients with severe symptoms, severe compromise in cardiac function and decompensation on recurrent basis, the term “advanced heart failure” is used [21]. There are non-specific symptoms of heart failure and thus differentiation of heart from other diseases based on these symptoms is difficult. Some specific signs, like apical impulse displacement, and raised jugular venous pressure, cannot be detected at ease [22]. In patients with other comorbidities like chronic lung disease or in obese patients or elderly ones, these signs and symptoms become hard to interpret [23] Iron is an essential element for almost all living organisms as it participates in a wide variety of metabolic processes, including oxygen transport, deoxyribonucleic acid (DNA) synthesis, and electron transport [24]. It is an integral part of hemoglobin (Hb) and myoglobin, acts as a co-factor in the biochemical pathway of oxidative metabolism and is involved in host defense mechanism [25]. Except for sloughing of duodenal enterocytes and bleeding, there is no natural mechanism for active excretion of iron. Therefore, regulation of iron balance occurs during intake, recycling and storage. Haem-specific receptor (HCP1), located in the duodenal enterocyte, absorbs organic haeme, which is then broken down intracellularly to yield biliverdin and free iron [26]. The divalent metal transporter 1 (DMT1) absorbs non-haeme inorganic iron, which exists in the trivalent ferric (Fe3+) form. This is then reduced by ferric oxidoreductases such as the duodenal cytochrome B, to the divalent ferrous (Fe2+) form before absorption from the apical surface of the enterocyte. Intracellularly, some of these ferrous ions conjugate with apoferritin to form ferritin, which remains within the enterocyte. The rest are converted back into their trivalent ferric state and excreted into the bloodstream via ferroprotein which is present in the basolateral surface of the enterocyte. This iron is then transported into the target tissues for storage and utilization [10]. Ferroprotein is a protein that exports iron from the cell, and is present in duodenal enterocytes, hepatocytes (which are the sites of iron storage) and in macrophages (which are the sites of iron recycling from senescent RBC’s). The function of ferroprotein is regulated by a liver-derived hormone called hepcidin, which is produced in response to elevated cytokine levels (especially IL-6) and fluctuating levels of iron within the hepatocyte [25]. Cardiac myocytes have high energy requirements, and they are therefore highly susceptible to iron deficiency and abnormal iron utilization [27]. It has been demonstrated that patients with end- stage heart failure who have been referred for cardiac transplantation, have depleted myocardial iron stores, as compared with healthy hearts. Also, soluble transferrin receptor (sTfR) levels were lower in the failing hearts that were explanted during transplantation, as compared with the levels in donor hearts that were deemed unsuitable for transplantation [28]. Exposure to aldosterone and noradrenaline, neurohormones that are commonly elevated in heart failure, resulted in a further reduction in the expression of soluble transferrin receptors (sTfR) [29]. Animal models have shown that sustained iron deficiency can result in left ventricular dilatation and hypertrophy, sarcomere disruption, mitochondrial swelling, and release of reactive oxygen species which can result in cell injury [30]. ID is the most common nutritional deficiency on the world. Basically, ID is linked to anemia but its role in the causation of HF or left ventricular dilatation has been ignored over past few years [7]. This study accessed the Iron Profile and vitamin B12 level in patients of HFrEF admitted in Manipal Teaching Hospital.
- MATERIALS & METHOD
A hospital based observational cross-sectional study was done for a period of fourteen months after ethical clearance. This study was conducted in Department of Internal Medicine of Manipal College of Medical Sciences, Pokhara Nepal. The hospital is the referral center for heart diseases from Western Regions of Nepal. This study considered 90% confidence interval with 10% margin of error Taking the prevalence as 30%84
Sample (N) = (1.6452 *P*Q)/E2
Where P = prevalence =30% (0.3) Q= 1-P=0.7
E= (margin of error) =10%
N= (1.6452*0.3*0.7)/0.12 = 56.8
Sample size: 56.8
At least 57 patients were included in my study. Data was collected and analyzed using statistical software SPSS version 25. All the patients diagnosed with HFrEF fulfilling the inclusion criteria, admitted in Cardiology unit of Manipal Teaching Hospital were included in the study after taking written consent from the patient / informant. The informants were clearly explained about the purpose and procedure of the study in native language. Detailed history was taken from the patient. General and systemic examinations was performed to look for signs of heart failure. Necessary blood investigations including iron profile (serum Iron, TIBC, serum ferritin, transferrin saturation) and vitamin B12 level was sent in all cases. Fresh peripheral venous blood samples were collected in ethylene diamine tetra acetic acid (EDTA) and serum tubes and sent for analysis. A complete hemogram with red cell indices was performed. Serum ferritin was measured using a fully automated bidirectionally interfaced Chemi luminescent immune assay. Serum iron was measured by the ferrozine method without deproteinization. Total iron binding capacity (TIBC) was measured by a spectrophotometric assay. Transferrin saturation was calculated as 100 x serum iron / TIBC. Vitamin B12 was measured by immunoassay. Transthoracic echocardiography was performed by cardiologists using Siemens Acuson, Model SC2000, 2D color Doppler. LVEF of ≤ 40% was included in the study. The proforma was filled by the researcher. The cases were recruited serially, until the required sample size was reached. All the analysis was performed using SPSS version 25. All the continuous variables were presented in frequency and percentage. Chi Square test and Fisher exact test were used as appropriate to test the significance. P value of ≤0.05 was considered statistically significant. No financial burden was given to the study population. Ethical clearance letter obtained from the Institutional Review Committee of Manipal College of Medical Sciences, Pokhara.
- RESULTS & DISCUSSION
Table 01: Age Distribution of Patient presenting with heart failure
| Age Range | Frequency | Percentage |
| 18 – 30 years | 3 | 5.3 |
| 31 – 50 years | 6 | 10.5 |
| 51 – 70 years | 20 | 35.1 |
| >70 years | 28 | 49.1 |
| Total | 57 | 100.0 |
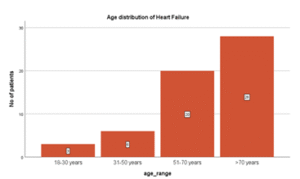
Figure 01: Age Distribution of Patient presenting with heart failure
In this study, maximum number of cases (almost 50%) belonged to age above 70 years. Mean age of cases included in my study was 64.95 ± 16 years
Table 02: Gender distribution of Heart Failure Patients with Reduced EF
| Gender | Frequency(n) | Percentage (%) |
| Male | 29 | 50.9 |
| Female | 28 | 49.1 |
| Total | 57 | 100% |
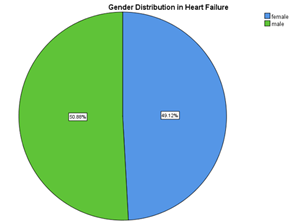
Figure 02: Gender distribution of Heart Failure
In this study, involvement of males and females were almost equal with % of male involvement being slightly greater.
Table 03: Gender distribution in Patients with Anemia
| Gender | Number of Anemic individuals (Cutoff values for male= <13g/dl) (Cutoff values for Female= <12g/dl) |
| Male | 15(44.1%) |
| Female | 19(55.9%) |
| Total | 100% |
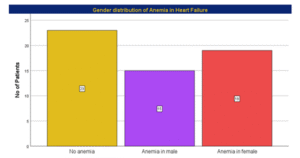
Figure 03: Gender distribution of Anemia
In this study, prevalence of anemia was 59.6%. Number of anemic males 44.1% and number of anemic females were 55.9%. Thus, this study showed number of females who had anemia were slightly higher than that of males. This finding was statistically significant (p < 0.001).
Table 04: Age distribution of Anemia
| Age range | Anemia in males | Anemia in Females | Total |
| 18 – 30 years | 2 (13.3%) | 0 (0.0%) | 2 (5.8%) |
| 31 – 50 years | 2 (13.3%) | 2 (10.5%) | 4 (11.8%) |
| 51 – 70 years | 5 (33.3%) | 6 (31.6%) | 11 (32.4%) |
| >70 years | 6 (40.0%) | 11 (57.9%) | 17 (50%) |
| Total | 15 (100%) | 19 (100%) | 34 (100%) |
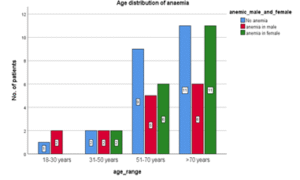
Figure 04: Age distribution of Anemia
In this study, it shows that percentage of anemic cases was higher in the older age groups. With half of the cases being above 70 years of age. However, it was not statistically significant. (P; 0.780).
Table 05: Age Distribution in Iron Deficiency Cases.
| Age range | Iron deficient cases (n) | % |
| 18 – 30 years | 1 | 3.3 |
| 31 – 50 years | 4 | 13.3 |
| 51 – 70 years | 9 | 30 |
| >70 years | 16 | 53.4 |
| Total | 30 | 100 |
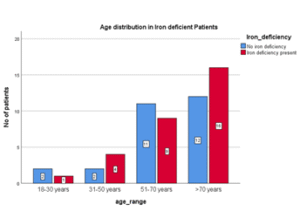
Figure 05: Age Distribution in Iron Deficiency Cases.
In this study, prevalence of iron deficiency was 52.6%. Number of iron deficient HFrEF cases were higher in older age groups. More than half of these cases were above 70 years age. However, the association was not statistically significant. (P= 0.681)
Table 06: Gender distribution in Iron Deficiency
| Gender | Iron Deficiency (n) | % |
| Male | 12 | 40 |
| Female | 18 | 60 |
| Total | 30 | 100 |
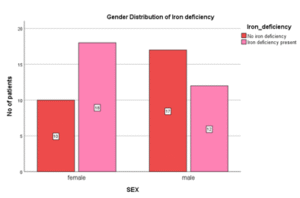
Figure 06: Gender distribution in Iron Deficiency
In this study, female iron deficient cases were higher (60%) compared to male. Thus, prevalence of iron deficiency in female was higher. It was however not significant statistically. (P= 0.083)
Table 07: Frequency of Anemia in Iron deficient cases
| Anemia | Non-Anemia | Total | |
| Iron Deficiency n | 20 | 10 | 30 |
| Iron Deficiency % | 66.7 | 33.3 | 100 |
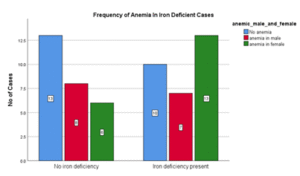
Figure 07: Frequency of Anemia in Patient who are Iron Deficient
In this study, out of 30 cases who were iron deficient, anemia was present in two third of cases (66%) and out of 27 non-iron deficient cases, prevalence of anemia was more than half (51 %.) There was no significant statistical association between anemia and iron deficiency in heart failure patients. (P= 0.236)
Table 08: Frequency of Iron Deficiency in Anemia
| Iron Deficiency present | No iron Deficiency | Total | |
| Anemia in male | 7 | 8 | 15 |
| Anemia in female | 13 | 6 | 19 |
| No Anemia | 10 | 13 | 23 |
| Total | 30 | 27 | 57 |
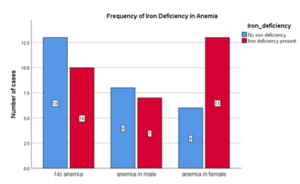
Figure 08: Frequency of Iron Deficiency in Anemia
Out of total anemic cases in this study, iron deficiency was present in more than half of cases (58%). In non-anemic cases, iron deficiency was present in 43%. There was no significant statistical association between anemia and iron deficiency in heart failure patients (P 0.236).
Table 09: Study of Serum Iron level
| N | Minimum | Maximum | Mean | Std Deviation | |
| S Iron ug/dl | 57 | 9 | 168 | 41.53 | 34.902 |
Mean value of Serum Iron was 41.53ug/dl with minimum and maximum value being 9ug/dl and 168ug/dl respectively. The standard deviation was found to be 34.902.
Table 10: Ferritin level and Left Ventricular Ejection Fraction
| Ferritin Level | ||||
| 0 – 99.9 | 100 – 1000 | Total | ||
| LVEF Range | 10 – 20% | 5 (33.3%) | 10 66.7% | 15 100% |
| 21 – 30% | 6 (27.3%) | 16 72.7% | 22 100% | |
| >30% | 4 (20%) | 16 80% | 20 100% | |
| Total | 15 (26.3%) | 42 (73.7%) | 57 100% | |
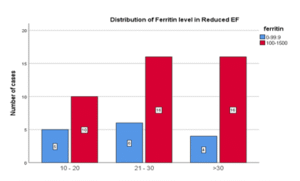
Figure 09: Ferritin level and Left Ventricular Ejection Fraction
In my study, in any range of ejection fraction, percentage of cases with the ferritin level <100 ng/ml was much lower compared to cases with ferritin level >100ng/ml There was no significant statistical association (P= 0.669) of ferritin level with HFrEF patients.
Table 11: Mean value of ferritin in HFrEF cases
| Ferritin ng/ml | Minimum | Maximum | Mean | Std. Deviation | N |
| Level of Ferritin | 23.9 | 1000 | 272.012 | 195.82 | 57 |
In this study, mean value of ferritin was 272 ± 195 ng/ml
Table 12: Transferrin saturation in patients with HFrEF
| Transferrin saturation | Frequency | Percentage |
| <20 | 43 | 75.4% |
| >20 | 14 | 24.6% |
| Total | 57 | 100% |
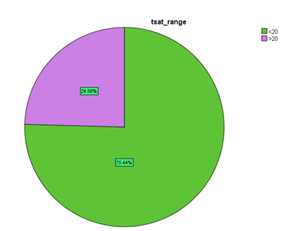
Figure 10: Showing percentage of patients with transferrin saturation in HFrEF.
Out of 57 patients enrolled in this study, it was found that 75.4% of patient had transferrin saturation less than 20%.
Table 13: Transferrin saturation level in Left Ventricular Ejection Fraction
| LVEF Range | T sat<20% | Tsat>20% | Total | |
| 10 – 20 | 13 86.7% | 2 13.3% | 15 | |
| 21 – 30 | 17 77.3% | 5 22.7% | 22 | |
| >30 | 13 65% | 7 35% | 20 | |
| Total | 43 | 14 | 57 |
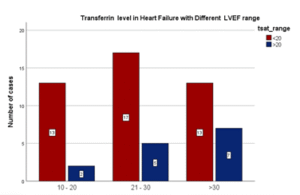
Figure 11: Transferrin saturation level in Left Ventricular Ejection Fraction.
In my study, almost 5 out of 6 cases (86.7%) with LVEF less than 20% had transferrin saturation <20%. Thus, percentage of cases with Transferrin saturation <20% increased as the LVEF declined. There was no significant statistical association of transferrin saturation level with different LVEF range in heart failure patients (p = 0.141).
Table 14: Comparison of Serum Ferritin with Transferrin Saturation level
| Ferritin < 100 | Ferritin≥100 | Total | |
| TSAT < 20% | 13 | 30 | 43 |
| TSAT ≥20% | 2 | 12 | 14 |
| Total | 15 | 42 | 57 |
Comparison between serum ferritin and transferrin saturation showed p value of 0.312. Hence the correlation of serum ferritin and transferrin saturation in HFrEF cases was not statistically significant (p >0.05) and were found to be independent of each other.
Table 15: Age Distribution in Vitamin B12 Deficiency
| Age range | Vitamin B12 deficiency | % |
| 18 – 30 years | 0 | 0 |
| 31 – 50 years | 3 | 19 |
| 51 – 70 years | 4 | 25 |
| >70years | 9 | 56 |
| Total | 16 | 100% |
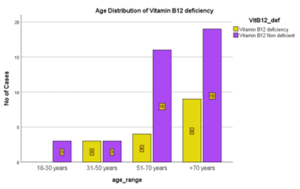
Figure 12: Age Distribution in Vitamin B12 Deficiency
In this study, prevalence of vitamin B12 deficiency was 28%. More than half of Vitamin B12 deficient cases were above age of 70 years (56%). Hence prevalence of Vitamin B12 deficiency was increasing with age. However, there was no significant statistical association between age and Vitamin B12 deficiency in heart failure patients. (p> 0.05) p value was 0.368.
Table 16: Gender Distribution of Vitamin B12 Deficiency
| Gender | Vit B12 Deficiency | VitB12 non-deficient | Total |
| Male | 4 (25%) | 25 | 29 |
| Female | 12 (75%) | 16 | 28 |
| Total | 16 (100%) | 41 | 57 |
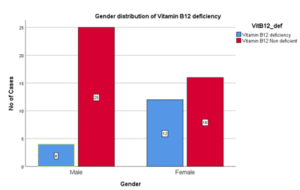
Figure 13: Gender Distribution of Vitamin B12 Deficiency
In this study percentage of males with vitamin B12 deficiency was 25%, [Females (75%)] Thus prevalence of Vitamin B12 deficiency was three times higher in females as compared to males and it was significant statistically. (P = 0.02).
Table 17: Frequency of Anemia in Vitamin B12 deficiency
| Vit B12 Deficiency | VitB12 Non deficient | Total | |
| Anemia in Male | 3 (18.75%) | 12 | 15 |
| Anemia in Female | 8 (50%) | 11 | 19 |
| Non-anemic Cases | 5 (31.25%) | 18 | 23 |
| Total | 16 (100%) | 41 | 57 |
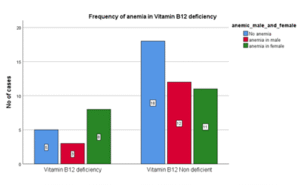
Figure 14: Frequency of Anemia in Vitamin B12 deficiency
In this study, anemia was present in 68.75% of vitamin B12 deficient cases. However, there was no significant statistical association between anemia and vitamin B12 deficiency in heart failure patients. (P: 0.247).
Table 18: Comparison of Iron Deficiency and Vitamin B12 deficiency with each other
| No Iron deficiency | Iron deficiency Present | Total | |
| Vitamin B12 Deficient | 5 | 11 | 16 |
| Vitamin B12 non-deficient | 22 | 19 | 41 |
| Total | 27 | 30 | 57 |
Comparison between Iron deficiency and Vitamin B12 deficiency showed p value of 0.128. Hence the co-existence of Iron and Vitamin B12 deficiency in HFrEF cases was not statistically significant.
DISCUSSION
Heart Failure is a clinical syndrome consisting of cardinal symptoms (e.g. breathlessness, ankle swelling, and fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles, and peripheral oedema). It is due to a structural and/or functional abnormality of the heart that results in elevated intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise [4]. Anemia is found to more prevalent in cases of heart failure. From the various studies it has been found that there is alteration in iron parameters in HFrEF cases. However, Vitamin B12 correlation with heart failure are still to be studied in detail. This study showed that the number of HFrEF cases increased with the advancement of age, highest prevalence being above age of 70 years. It could possibly be because of multiple co morbidities that occurs in elderly and aged populations. In this study, the mean age of patients with heart failure was, 68.95 ± 16 years. In similar study, the mean age of heart failure patients was 64.52 ± 14.69 years [31]. In this study, the percentage of males and females were almost equal. (Male 50.9%, Fe- 49.1%). In similar study, the percentage of male and female involved was 49% and 51% respectively [32]. In our study, out of 57 patients, 34 (59.6%) patients had anemia. The number of females with anemia was slightly higher (55.9%). In similar study, the prevalence of anemia was 48.33% [31]. The cause of anemia in patients with heart failure can be due to expansion of plasma volume or decreased RBC mass causing patient to be anemic. Expansion of plasma volume has also been found to increase natriuretic peptides which define why heart failure patients have low hemoglobin for a given level of iron deficiency. Addition of erythropoietin improving both anemia and heart failure signifies their relationship [32]. From the findings of this study, total iron deficient cases were more than half of the total cases (52%). In similar study, prevalence of iron deficiency in heart failure cases were almost similar to my study. (50%) [33]. A Study, showed 46.67% of iron deficient cases, the value is almost similar as compared to my study [31]. In other study 37% of all chronic systolic heart failure patients were Iron deficient [28]. In the study, 36% of cases had iron deficiency [24]. The number of Iron deficient cases increased with the advancement of the age. High prevalence of iron deficiency in heart failure patients especially in elderly in our country as compared to study done in other countries could be due to poor diet and poor nutrition status of people and diminished iron absorption and gastrointestinal blood loss in chronic heart failure cases [34]. From findings of this study, the prevalence of Anemia and Iron Deficiency in HFrEF cases were almost equal (59.6% vs 52%) and similar result was found in study done by [31]. This study showed female iron deficient cases were much higher (60%). This finding supports the findings from other studies which showed female gender as an independent correlate of iron deficiency in heart failure [35] Menstrual bleeding as a cause for high prevalence of iron deficiency in the female heart failure patients is less likely because all of the female subjects were post menstrual. This study showed prevalence of anemia in iron deficient cases to be 66.7%. In study conducted by an author, there was no increased frequency of anemia in iron deficient cases [20]. The possible cause of increased prevalence of anemia in Iron deficient Heart failure cases in my study could be due malnutrition and inadequate knowledge on balanced diet of people in our setting. In this study, prevalence of iron deficiency in anemia case was 58%, which is higher than prevalence of iron deficiency in non-anemia cases (43%). In the study [36] prevalence of iron deficiency in anemic vs non anemic cases were 57% and 32% respectively which was almost similar to the findings of this study. In another study, similar results were found. Prevalence of Iron deficiency was higher in anemia with heart failure cases (78%) compared to that in non-anemia cases (65%) [37]. However, the overall prevalence in each class (anemia vs non anemia) was higher compared to this study. It could be because it involved only chronic heart failure cases and the anemia in part was contributed by anemia of chronic disease. But our s study and study [36] included acute and chronic heart failure patients. In this study 75.4% of cases had Transferrin saturation of <20%. Percentage of cases with Transferrin saturation <20% increased as the LVEF declined. However, it was not statistically significant (p = 0.141). There are limited studies that had been conducted to depict relationship of transferrin saturation with heart failure. A study done by Andrew et al, showed that TSAT <20% was independently associated with an increased risk of HF hospitalization regardless of serum ferritin level, which looks somehow similar to this study where three fourth of patient with heart failure have transferrin saturation <20% and all of these cases were hospitalized due to symptoms of heart failure. The reason behind the correlation can be compared to the reason by which iron deficiency and HF are correlated [38]. In this study it was found that most of the cases with HFreF had ferritin level more than 100, in each range of ejection fraction. The mean value of ferritin was 272 ± 195 ng/ml. Although HFrEF cases had higher prevalence of anemia, patients who showed LV systolic dysfunction were less likely to have a low serum ferritin concentration in comparison to those without HF. In similar study [39], he concluded that level of serum ferritin had no significant correlation with HFrEF [40] which was similar to the findings of this study. Out of total 57 HFrEF cases enrolled in this study, total Vitamin B12 deficient cases were 16 (28%). It was much lower in value as compared to iron deficiency. (52%). In study [41], prevalence of B12 deficiency was much lower as compared to iron deficiency anemia which matched to this study. The reason behind less significance of B12 deficiency in heart failure can be attributed to blood congestion in the liver caused by heart failure giving rise to hepatic cell damage and mild liver dysfunction leading to the release of hepatic and metabolically inactive vitamin B12 analogues, thus making serum vitamin B12 measurements unreliable [42].
- CONCLUSIONS
This was a hospital based observational cross-sectional study done for a period of fourteen months after ethical clearance. A total of 57 patients who had a diagnosis of heart failure with reduced ejection fraction were studied. Prevalence of heart failure was much higher in elderly >70 years and male and female heart failure cases were almost equal in number. Prevalence of iron deficiency in heart failure patients was found in 52% and it showed female preponderance but was not significant statistically. The prevalence of iron deficient cases increased with the age and more than half of cases with iron deficiency were above 70 years age. However, it was not statistically significant. Anemia was seen in more than half of total HFrEF cases (59.6%). Anemic females were more than anemic males, and it was found to be statistically significant. The prevalence of anemia was increasing with age. However, there was no significant correlation between age and anemia in HFrEF cases. In this study, sixty six percent of iron deficient cases were anemic while 51% of iron non deficient cases were anemic. Similarly iron deficiency was seen in 58% of anemic cases and 43% of non-anemic cases. However, the association between anemia and iron deficiency in heart failure patients was found to be statistically non-significant. Majority of HFrEF cases had low serum iron level however serum ferritin was found on higher side in most of HFrEF cases. There was no any significant relationship between ferritin and LV ejection fraction. Three fourth of patients had transferrin saturation less than 20% and cases with declining EF had very low TSAT (<20%). It was also found that there was no significant correlation between transferrin saturation and ferritin level as these parameters are independent variables. Prevalence of vitamin B12 deficiency was more than one fourth of cases. The elderly people had highest prevalence of vitamin B12 deficiency however it was not statistically significant. Prevalence of Vitamin B12 deficiency was three times higher in females as compared to males and it was found to be significant statistically. Anemia was found in two third of vitamin B12 deficient cases however it was not statistically significant. There was no significant correlation between vitamin B12 deficiency and iron deficiency in heart failure. Iron deficiency was prevalent in patients with heart failure and reduced ejection fraction irrespective of anemia and hemoglobin levels. Measurement of iron status could be considered during workup of heart failure patients. Statistically significant association with anemia was detected in female gender. The association of Ferritin level with HFrEF was not statistically significant. Transferrin saturation was low in most of the cases of HFrEF. Contrary to our expectation, Vitamin B12 deficiency prevalence was relatively low in our study and statistically significant association was seen in female population. Further studies are required to assess the prognostic value of iron parameters measurement and influence the treatment of iron deficiency in heart failure patients.
REFERENCES
- Inamdar AA, Inamdar AC. Heart failure: Diagnosis, management and utilization. J Clin Med. 2016;5(7):62.
- Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358(20):2148–59.
- Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview.Heart. 2004;90(4):464–70.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
- Raksha Rimal, Anu Kafle, Arju Sah, Ankur Bista, Anshu Yadav & Sampada Khatiwada (2024). Comparative Study of Hematological Parameters among First-Time Blood Donors & Repeat Blood Donors of Kathmandu Valley. Dinkum Journal of Medical Innovations, 3(04):302-312.
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34(11):816–29.
- Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124(20):2253–63.
- Blanc B, Finch CA, Hallberg L, et al. Nutritional anaemias. Report of a WHO Scientific Group. WHO Tech Rep Ser. 1968;405: 1-40.
- Sandra Rumi Madhu (2024). A Study on Anemia in Adolescent Girls Due to Food Habit at Gazipur District in Bangladesh. Dinkum Journal of Medical Innovations, 3(06):469-482.
- Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008;83(11):1203–12.
- Davis RC. ABC of heart failure: History and epidemiology. BM. 2000;320(7226):39–42.
- Norman J.N. William Withering and the purple foxglove: a bicentennial tribute. J ClinPharmacol. 1985;25:479–483.
- Wang TJ. Natural history of asymptomatic left ventricular systolic dysfunction in the Circulation 2003;108:977–982.
- Nabin Kumar Sinjali Magar, Dr. Dhruba Gaire & Dr. Prasanna Bahadur Amatya (2024). Evaluation of Pulmonary Hypertension in Chronic Obstructive Pulmonary Disease (COPD) by assessment of Chest X- Ray, ECG and Echocardiography. Dinkum Journal of Medical Innovations, 3(02):132-144.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. J Am CollCardiol. 2013;62(16):147–239
- Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9(6–7):684–94.
- Killip T III, Kimball JT. Treatment of myocardial infarction in a coronary care unit. Am J Cardiol. 1967;20(4):457–64
- Becher PM, Lund LH, Coats AJS, Savarese G. An update on global epidemiology in heart failure. Eur Heart J. 2022;43(32):3005–7.
- Harikrishnan S, Jeemon P, Ganapathi S, Agarwal A, Viswanathan S, Sreedharan M, et al. Five-year mortality and readmission rates in patients with heart failure in India: Results from the Trivandrum heart failure registry. Int J Cardiol. 2021;326:139–43.
- Koirala B, Dennison Himmelfarb CR, Koirala B, Budhathoki C, Davidson PM. Epidemiology and management of heart failure in Nepal. J Card Fail. 2019;25(10):846– 8.
- Hawkins NM, Petrie MC, Jhund PS, ChalmersGW, Dunn FG, McMurray JJV. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail 2009;11:130–139.
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health.Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences. 2014;19(2):164.
- Sazzad Hossain, Dr. Deb Dulal & Dr. Fuad Faysal (2024). Prevalence of Cardiovascular Disease and Associated Risk Factors among Adults. Dinkum Journal of Medical Innovations, 3(05):379-390.
- Chua AC, Graham RM, Trinder D, Olynyk JK. The regulation of cellular iron metabolism. Crit Rev Clin Lab Sci 2007;44:413–459.
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acutephase protein. Blood 2003;101:2461– 2463.
- Suzuki T, Hanawa H, Jiao S, et al. Inappropriate expression of hepcidin by liver congestion contributes to anemia and relative iron deficiency. J Card Fail 2014;20:268–277.
- Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010;31:1872– 1880.
- Leszek P, Sochanowicz B, SzperlM, Kolsut P, Brzoska K, Piotrowski, et al. Myocardial iron homeostasis in advanced chronic heart failure patients. Int J Cardiol 2012;159:47–52.
- Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol 2011;58:474–480.
- Dong F, Zhang X, Culver B, Chew HG Jr, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. ClinSci (Lond) 2005;109:277–286.
- Yeo TJ, Yeo PS, Ching-Chiew Wong R, Ong HY, Leong KT, Jaufeerally F et al, Iron deficiency in a multi-ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014;16:1125–1132.
- Rangel I, Goncalves A, de Sousa C, Leite S, Campelo M, Martins E, et al, Iron deficiency status irrespective of anemia: a predictor of unfavorable outcome in chronic heart failure patients. Cardiology 2014;128:320–326.
- Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, von Haehling S, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013;34:827–834.
- Mahaseth A, Shah JN, Nepal B, et al. Prevalence and pattern of Iron deficiency in patient with heart failure with reduced ejection fraction. Janaki MedColl J Med Sci. 2019;7(2):10–6.
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575-582.
- Silvestre OM, Gonçalves A, Nadruz W Jr, et al. Ferritin levels and risk of heart failure-the Atherosclerosis Risk in Communities Study. Eur J Heart Fail. 2017;19(3):340–7.
- Negi PC, Dev M, Paul P, Pal Singh D, Rathoure S, Kumar R, et al. Prevalence, risk factors, and significance of iron deficiency and anemia in nonischemic heart failure patients with reduced ejection fraction from a Himachal Pradesh heart failure registry. Indian Heart J. 2018;70(Suppl 3):S182–8.
- Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, et al. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart 2002;87:235–241.
- Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, et al. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol 2014;174:268–275.
- Comin-Colet J, Enjuanes C, Gonzalez G, et al. Iron deficiency is a key determinant of health- related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013;15:1164–1172.
- Cleland JGF, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, et al. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol. 2016;1(5):539.
- Bistola V, Filippatos G. Vitamin B12 deficiency in heart failure: another “brick in the wall.” Hellenic J Cardiol. 2020;61(5):338–40.
Publication History
Submitted: February 19, 2025
Accepted: March 02, 2025
Published: April 30, 2025
Identification
D-0418
DOI
https://doi.org/10.71017/djmi.4.4.d-0418
Citation
Bibek Lamichhane (2025). Study of Iron Profile and Vitamin B12 in Patients with Heart Failure with Reduced Ejection Fraction. Dinkum Journal of Medical Innovations, 4(04):147-164.
Copyright
© 2025 The Author(s).


