Publication History
Submitted: October 31, 2024
Accepted: November 22, 2024
Published: March 31, 2025
Identification
D-0412
DOI
https://doi.org/10.71017/djnsi.4.3.d-0412
Citation
Venus O. Saz & Hamsha R. Saz (2025). Application of Soil Amendments on the Chemical Fractions of Chromium and Nickel in Lowland Rice Soils as Affected by Mining Activities Dinkum Journal of Natural & Scientific Innovations, 4(03):195-209.
Copyright
© 2025 The Author(s).
195-209
Application of Soil Amendments on the Chemical Fractions of Chromium and Nickel in Lowland Rice Soils as Affected by Mining ActivitiesOriginal Article
Venus O. Saz 1*, Hamsha R. Saz 2
- Associate Professor, Cavite State University, Indang, Cavite, Philippines.
- Associate Professor, Cavite State University, Indang, Cavite, Philippines.
* Correspondence: vosaz@cvsu.edu.ph
Abstract: The presence of Cr and Ni in agricultural land particularly in rice paddies affected by mining activities represents a major risk to the environment and human health. Thus, remediation techniques such as the application of soil amendments are very important to mitigate the availability of these metals. The study was conducted to assess the influence of soil amendments application on the chemical fractions of Cr and Ni in the soil and to evaluate its efficacy in mitigating the absorption and translocation of these metals and at the same time increase rice productivity in the affected area of Santa Cruz, Zambales. The experimental set up was applied with vermicast, bagasse ash, carbonized rice hull and zeolite at 10 t ha-1 in combination with the recommended rate of fertilizers for two cropping seasons. Results showed that all soil amendments reduced the total Cr and Ni. Among the treatments, zeolite contributed the highest reduction in Cr followed by vermicast, bagasse ash and carbonized hull. However, bagasse ash constitutes the highest reduction of Ni followed by zeolite, vermicast, and carbonized rice hull. The application of soil amendments proved efficient in preventing bioaccumulation and translocation of Cr and Ni in rice, due to the binding or adsorptive capacity of these materials that helped reduce dissolved metal concentrations. After each cropping season, Cr was present only in three soil fractions which are stable, immobile and unavailable arranged in the following decreasing order: residual>bound to organic matter>bound to Fe-Mn oxides while Ni was detected in more fractions, residual > bound to Fe-Mn oxide > bound to organic matter > exchangeable form > bound to carbonate. Both Cr and Ni have low mobility factor indicating that the mobility and biological availability of these metals were relatively low and therefore has low potential risk to the environment compared to no soil amendments applied.
Keywords: contaminants, mitigation, heavy metals, fractionation, bioaccumulation
1. INTRODUCTION
In the modern world, soil and agricultural pollution brought on by mining operations is a significant problem [1]. Mica deposits in India cover a total area of about 3888 km2 in the districts of Giridih and Koderma in Jharkhand and Munger in Bihar. The majority of mines in this area are still active right now, but a few have been closed for several years. Mica wastes from these mines (active and closed) are typically fine, loose, and homogeneous, have a low bulk density and moisture-holding capacity, and lower nutrients, which not only inhibit plant colonization but also contaminate the surrounding areas during strong winds and heavy runoff during monsoon [3]. Around 75% of raw mica mine waste is disposed of nearby the mines during the dressing process, and as a result, these mine wastes wash into the fields and rivers, contaminating land and water resources . Rice is the major crop in the mica mine waste-contaminated soils in that region. In India, daily consumption of milled rice is high, approximately 103 kg per capita/per year. Rice is prone to absorb some toxic heavy metals such as cadmium (Cd), chromium (Cr), nickel (Ni) and lead (Pb), which is a major threat to food security and human health [5]. Increased accumulation of Cd in rice as a result of extensive Cd contamination in paddy soils brought on by mining, smelting, and other industrial operations has been reported by. Lead contamination in rice due to mining activities have been previously reported in Zamfara state, Nigeria. Although the total metal content in the soil is an effective indicator of soil pollution, it does not provide adequate information about the potential environmental impact. Many studies have demonstrated that heavy metal uptake by plants is positively related to the bioavailable concentration of soil heavy metals. Sequential chemical extraction methods are more popular to use quantification of different fractions of metal in soil [7]. The sequential chemical extraction technique breaks down metals into different solubility and mobility forms and can be used to predict the conversion of metals into different fractions of metals in soils and the availability of metals in microorganisms. Several workers studied the effect of different metal fractions on soil microorganisms and metal uptake by rice plants in mining-affected soils [4].Soils polluted with heavy metals like chromium (Cr) and nickel (Ni) have become common across the globe due to increase in geologic and anthropogenic activities like mining. In fact, the municipality of Santa Cruz is primarily an agricultural town due to its rich arable lands that are conducive for farming. However, mining industry has recently been booming in the area where there is an abundant deposit of chromium and nickel laterite ores. The Center for Environmental Concerns [1] reported that agricultural production in the municipality has been affected since mining operations started in 2006. People attribute the diminishing production due to the presence of these heavy metals, which had contaminated their rice fields, waterways and coastline. Farmers’ harvest had gone down from an average of 100 to 120 cavans per hectare in 2009, to only 75 to 80 cavans because of the chromium and nickel laterite-laden soil was hard to till, and water was hard to permeate, so the rice plants growth was stunted because high heavy metal concentrations retard shoot and root growth, deform various plant parts, decrease biomass production, disturb mitotic root tips, and produce nutrient deficiency that leads to chlorosis and foliar necrosis. Additionally, excess Cr and Ni also affects nutrient absorption by roots, impairs plant metabolism, inhibit photosynthesis and transpiration [2]. Farmers tried to remedy this by applying more fertilizer, which jacked up their production costs, but hardly made a difference. The processing of these metal elements in the municipality has become a major source of farmland heavy metal contamination [3] that leads to potential environmental threat to the safety of agricultural crops such as rice which is consumed by majority. Plants growing on this soil suffer metal toxicity that results to reduction of growth performance, and yield as a result of changes in physiological and biochemical processes in plants. Continued decline in plant growth reduces yield which eventually leads to food insecurity. Knowledge of the total metal concentration provides limited information about their potential mobility and bioavailability. However, fractionation of heavy metals like Cr and Ni helps to determine their binding form, toxicity, and availability in terrestrial environments. According to [5] fractionation involves successive extraction steps using chemical reagents of different binding strengths and metal specificity to destroy the bond between the metals and inorganic specific fraction of the soil. This also determine both the actual and potential mobility of metal elements in soils which classifies them into the following five fractions namely: exchangeable, carbonate, Fe/Mn oxides, organic and residual fractions. It is well known that the degree of heavy metal pollution in the soil depends more on the chemical forms than the total metal concentration. Thus, it becomes a matter of urgency to determine the chemical fractionation of Cr and Ni and to find materials to remove or minimize the concentration of these pollutants. The identification of the chemical forms of Cr and Ni in the rice paddies are necessary for estimating their biological availability, physio-chemical reactivity and transport in the environment and food chain in sustaining soil health, ensure food safety and productivity. There is no available information yet on the distribution pattern of Cr and Ni among various chemical species or soil fractions in lowland rice soils of Santa Cruz, Zambales, hence this study was conducted.
2. MATERIALS AND METHODS
A field experiment was conducted from June 2017 to February 2018 in Barangay Lomboy, Sta. Cruz, Zambales. The geographical location of the study area is 15o46’38.1″N latitude and 119 o56’14.1″E longitude with elevation of 11.8 m above sea level (Figure 01).
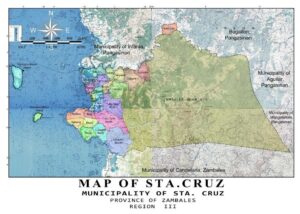
Figure 01: Brgy Lomboy, Santa Cruz, Zambales Map showing the location of the study site
2017 WS. For the first cropping season, rice area was treated with soil amendments (vermicast, carbonized rice hull, bagasse ash, and zeolite). All cultural management practices (fertilizer rate, weeding, pest control) were based on the Rice Crop Manager (IRRI 2013). Soil amendments were applied at a rate of 10 t/ha. Each block has a dimension of 4m x 5m and spaced at 0.50m from each other. All plots were separated by small bunds and canals to avoid contamination of treatments. The different treatments were laid out in Randomized Complete Block Design (RCBD) with four replications. NSIC Rc160 (Tubigan 14) rice variety was planted. The experimental layout is shown in Figure 02. The details of the treatments were as follows:
T1- Farmer’s practice (No soil amendment)
T2- Farmer’s practice + Vermicast
T3- Farmer’s practice + Carbonized rice hull
T4- Farmer’s practice + Bagasse ash
T5- Farmer’s practice + Zeolite
All plots received 82-16-16 kg N- P2O5 – K2O/ ha based on Rice Crop Manager (IRRI 2013). During basal application, 2.24 bags (112 kg/ ha) of 14-14-14 (complete fertilizer) was used. Second application was at 21-25 DAT with 1.43 bag (71.74 kg/ ha) of urea (46% N) and 3rd application was at 35-39 DAT with 1.43 bags (71.74 kg ha-1) of urea (46% N). 2018 DS. During the second cropping season the same area were grown with rice using RCM as basis of fertilization. Rice was treated again with the same soil amendments except that higher rates of NPK were applied. The experimental layout is shown in Figure 3. The details of the treatments were as follows:
T1 – Farmer’s Practice (No soil amendment)
T2 – Farmer’s practice + vermicast
T3 – Farmer’s practice + carbonized rice hull
T4 – Farmer’s practice + bagasse ash
T5 – Farmer’s practice + zeolite
All plots received 129-36-46 kg/ ha N-P2O5-K2O, based on Rice Crop Manager procedure. During basal: 5.14 bags (257.14 kg/ha) of 14-14-14 (complete fertilizer) were applied. Second application was 21-25 DAT: 2.02 bag (101. Kg/ ha) of 46-0-0 (urea). Third application was 35-39 DAT: 2.02 bags (101 kg ha-1) of 46-0-0 (urea) 0.33 bag (16.67 kg/ ha) of 0-0-60 (muriate of potash).
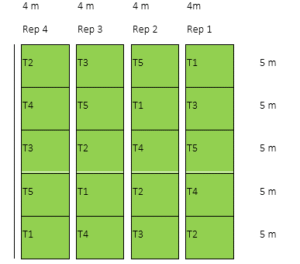
Figure 02: Experimental lay-out for the first cropping
Legend:
TI – Control/Farmer‘s practice T4- Bagasse ash
T2- Vermicast T5- Zeolite
T3- Carbonized rice hull
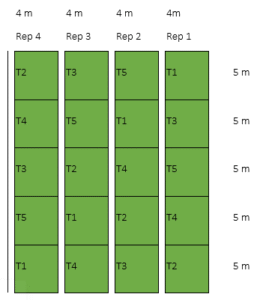
Figure 03: Experimental lay-out for the second cropping
Legend:
T1 – Control/Farmer’s practice
T2 – Vermicast
T3 – Carbonized rice hull
T4 – Bagasse ash
T5 – Zeolite
The experimental field was tilled before planting with a moldboard plow followed by two passes of a leveling disk with the first one done soon after the moldboard plow tillage and then shortly before transplanting. Bunds and drainage canals were constructed around the experimental field. Final leveling was done after puddling the soil. Sprouted rice seeds at 60 kg/ ha NSIC Rc160 (Tubigan 14) were sown on a nursery seedbed to raise the seedlings. Fertilizers were applied based on the recommended rate of the Rice Crop Manager (RCM) for both cropping seasons. Organic soil amendments were applied 7 days before transplanting at a rate of 10t /ha. Seventeen to twenty-day old seedlings were transplanted in the field on June 2017 (WS)) and November 2017 (DS) at 0.20m x 0.20m planting distance with 2-3 rice seedlings per hill. Missing hills were replaced during the first two weeks after transplanting to ensure uniform crop stand. All plots were kept weed-free during the growing season, particularly before each fertilizer application. Pests and insects were controlled according to the best management practices for irrigated rice production. The crop was supplemented with irrigation water when necessary. Excessive water and water deficit that could affect the growth and yield of the crop was avoided. Intermittent or controlled irrigation system was employed. Water depth of 3–5 cm was maintained at every irrigation time from early tillering until 1-2 weeks before crop maturity or harvest. Soil samples were collected before and after harvesting for two (2) cropping seasons. For each sampling, about one kilogram of composite soil sample was collected from the top 20cm layer using soil auger and placed in a properly labeled plastic container. Monthly averages of rainfall, and temperature were obtained from PAG-ASA weather station in Iba, Zambales. Modified Sequential Extraction Procedure by Tessier [11] was used to determine the various fractions of Cr and Ni in soil which include: water soluble, exchangeable, bound to carbonates, bound to Fe-Mn oxides, bound to organic matter, and residual. The quantities indicated below refer to 1-g soil samples (dry weight of the original sample used for initial extraction). Water Soluble: Soil sample was extracted at room temperature with 8mL deionized water for 2 hours with continuous agitation. Exchangeable. The soil was extracted at room temperature for 1 hr. with 8 mL of either magnesium chloride solution (1 M MgCl, pH 7.0) agitation. Bound to Carbonates. The residue from (2) was leached at room temperature with 8 mL of 1 M NaOAc adjusted to pH 5.0 with acetic acid (HOAc). Continuous agitation was maintained. Bound to Fe-Mn Oxides. The residue from (3) was extracted with 20 mL of 0.3M Na2S2O4 + 0.175 M Na-citrate + 0.025 M H-citrate, as prescribed by [12]. The latter experiments were performed at 96 + 30 with occasional agitation and the time needed for complete dissolution of the free iron oxides was evaluated. Bound to Organic matter. A method described by Gupta and Chen (1975) was adopted. The residue from (4) was mixed with 3 mL of 0.02 M HNO3 and 5 mL of 30% H2O2 adjusted to pH 2 with HNO3, and the mixture was heated to 85+ 20 C for 2 hr. with occasional agitation. A second 3-mL aliquot of 30% H2O2 (pH 2 with HSO,) was added and the sample was heated again to 85+ 2 0C for 3 hrs with intermittent agitation. After cooling, 5 mL of 3.2 M NH4OAc in 20% (v/v) HNO3 was added and the sample was diluted to 20 mL and agitated continuously for 30 minutes. The addition of NH4OAc was designed to prevent adsorption of extracted metals onto the oxidized sediment. Residual. The residue from (5) was digested with a 5:1 mixture of HF- then dissolved in 12N HCl and diluted to 25 ml. The resulting solution was then analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES) for Cr and Nis using the standard addition technique. The soil samples were digested using Milestone Ethos UP Microwave Digestion System, a microwave-assisted extraction system which allows trace metals to be extracted from various matrices such as soil, plants, food etc. for subsequent analysis by ICP-OES or AAS. Compared with hot-plate digestion, microwave digestion has the following advantages: the digestion time is shorter, sufficient digestion of samples, reagent consumption is lower, as well as good accuracy and repeatability. Using an analytical balance, 0.50 grams of soil samples were accurately weighed, and transferred into the microwave PTFE vessels. Concentrated nitric acid and 30% hydrogen peroxide were added inside each vessel at a ratio of 7:3 (v/v). The PTFE vessels were hermetically sealed, then positioned inside the digester. The digestion program consisted of a 20-min gradual increase in temperature to 200°C, a 20-min isothermal step at 200°C (1800 W) and then a ventilated cooling stage. After cooling to room temperature, all the digests were filtered through a Whatman filter paper (grade 42), then collected in plastic vials. The concentration of the Cr, Ni, and other trace metals in the digestion solution was measured using a Teledyne Leeman Labs Prodigy 7 ICP-OES equipped with quantification software for simultaneous measurement of all analyte wavelengths of interest. Quality Control (QC) samples were analyzed to check the accuracy and precision of the calibration curve for each element. Concentrations of trace metals were reported as µg g-1 soil (ppm). The early fractions, water soluble, exchangeable and carbonate (water soluble + Exchangeable + Carbonate) are regarded as the most reactive, most mobile and most potentially available/bioavailable fractions. The metals in these fractions consist of those that can be accessed by man through ingestion and are usually considered as being of anthropogenic origin [13]. The metals in these phases are weakly or loosely bound to soil components and the relative index mobility of these metals was calculated as mobility factor (MF) using the following equation.
MF = ————————————– X 100
Where:
F1 = water soluble
F2 = exchangeable
F3 = carbonate
F4 = bound to Fe Mn
F5= bound to Organic Matter
F6 = residual
Grain Yield. It was taken from 5 m2 (2.5 m x 2.0 m) sampling area located at the center each plot. Grain yield was adjusted to 14% MC, and at per hectare basis using the formula:
Yield sample (kg) 10,000 m2 100-MC
Yield (kg ha-1) = ———————— × ————– × ————–
Sampled area (m2) 1.0 ha 86
Plant samples were collected at harvest. Samples were cleaned from dust and soil particles or any contamination then oven dried at 700 C to minimize loss of soluble constituents and to avoid thermal decomposition of the material. Oven-dried tissue samples were ground and placed in a properly labelled coin envelope and stored in a desiccator.
The bioaccumulation factor (BAF), is an indicator of the ability of a plant to accumulate a specific metal and was calculated using the formula:
C plant
BAF = ———–
C soil
where the heavy metal concentration in the edible parts of the plant and the soil is represented by C plant and C soil, respectively. To establish the relative translocation of metals from the soil to grains, the Translocation Factor (TF) or mobilization ratio was calculated using the formula:
Conc. of metal in grains
TF = ——————————–
Conc. of metal in soil
The soil type in the area is Bani silty clay with initial soil characteristics presented in Table 01. The area has an adverse soil fertility condition as indicated by its strongly acidic pH (5.1) and low SOM content (1.76 %). It also contains very high concentrations of total Cr (2 191 mg/ kg) and total Ni (1 510 mg/ kg) that may have resulted from the weathering of parent material composed of ultramafic rocks rich in mineral resources such as Ni, chromite, Fe and Mn [15]. Improper disposal of mining waste from chromite and nickel mines might also have resulted in groundwater contamination or seepage in the area. Moreover, the continuous use of irrigation water contaminated with Cr (<0.05 mg/ kg) and Ni (0.019 mg/ kg) has also aggravated the situation. According to many researchers the availability of heavy metals in soil is associated with several environmental soil factors including pH, soil organic matter content (SOM), and (CEC) cation-exchange capacity [17].
Table 01: Soil property
| SOIL PROPERTY | Initial |
| Soil pH (H2O,1:1) | 5.1 |
| Soil organic matter (%) | 1.76 |
| Total Cr Content (mg /kg) | 2,191 |
| Total Ni Content (mg /kg) | 1,510 |
As shown in Table 02, after one cropping season (2017 WS), soil total Cr was relatively higher in the non-amended soil (control) with 2 057 mg/ kg followed in decreasing order by carbonized rice hull (2 030 mg/ kg) > bagasse ash (1 998 mg/ kg) > vermicast (1 956 mg/ kg) > zeolite (1 918 mg /kg). Carbonized rice hull is made from incomplete burning of rice hull and this material is widely used as soil conditioner and seedling media because its loose, porous and contains phosphorous, potassium, calcium and other micronutrients. With it being a burned material, this contains significant amounts of carbon which is known as an adsorbent element. Bagasse ash, a completely burned material from extracted sugarcane stalks, contains high amount of silica that is characterized as adsorbent compound and remained organic matter. Vermicast is a material that has been widely used as organic matter source or soil conditioner and was reported by [18] to cause significant reduction of heavy metals in soils. Moreover, zeolite, a naturally occurring compound, is known to have high degree of ion exchange characteristics, it has the ability to adsorb high amounts of heavy metals in the soil reducing total Cr content to 1 918 mg/ kg.
Table 02: Soil total Cr and Ni concentrations (mg/ kg) after 2017 WS and 2018 DS
| Treatments | Chromium (mg/ kg) | Nickel (mg/ kg) | ||
| 2017 WS | 2018 DS | 2017 WS | 2018 DS | |
| Control | 2,057 | 2,031 | 1,492 | 1,352 |
| Vermicast | 1,956 | 1,848 | 1,444 | 1,248 |
| Carbonized Rice Hull | 2,030 | 2,019 | 1,357 | 1,257 |
| Bagasse Ash | 1,998 | 1,986 | 1,374 | 1,002 |
| Zeolite | 1,918 | 1,841 | 1,380 | 1,062 |
Total level of Cr in the soil continued to decrease after 2018 DS including in the non-amended soil (control) with 2 031mg/ kg due to less precipitation / water that may have led to less solubility of elements particularly heavy metals. However, it was observed that plots added with soil amendments showed lower amounts of Cr compared to farmer’s practice (control). Among the soil amendments, zeolite had the lowest soil total Cr with 1 841 mg/ kg as shown in Table 02. This implies that zeolite is efficient in mitigating Cr in the soil due to its adsorptive property [21]. Soil total Ni after 2017 WS was relatively higher in the non-amended soil (control) with 1 492 mg/ kg followed in decreasing order by vermicast with 1 445 mg/ kg, zeolite with 1 380 mg/ kg, bagasse ash with 1 374 mg/ kg and carbonized rice hull had the least total Ni content with 1 357 mg /kg. The different soil ameliorants can reduce total Ni content in the soil as shown in Table 2 due to their excellent affinity for metal cations as adsorptive and catalysts property that can immobilize or precipitate the metal pollutants in the soil. The total Ni content after 2018 DS was reduced further with bagasse ash which resulted in highest reduction (1 003 mg/ kg) while control still had the least reduction (1 352 mg/ kg). After 2017 WS, Cr was found only in the residual fractions, bound to organic matter and Fe-Mn as shown in Table 3. Results showed that Cr is highly concentrated in the residual fraction compared to the other fractions indicating that it is stable in this form. Cr fraction in all the treatments were found in the order: residual fraction > fraction bound to organic matter > fraction bound to Fe/Mn oxides. Among the fractions only Fe/Mn and organic matter show significant amounts of Cr in all treatments. Cr was below detectable levels in fractions bound to carbonates, exchangeable and water-soluble fractions which are the relatively available forms. [19] reported that when there are changes in the redox status of the soil, heavy metals become available in form.
Table 03: Total concentration (mg/kg) and percentage distribution of Cr in different fractions as influenced by soil amendments (2017 WS)
| Treatments | Soil Fractions | |||||
| Residual | Bound to OM | Bound to Fe/Mn oxide | ||||
| Total concentration | Percentage Distribution | Total concentration | Percentage Distribution | Total concentration | Percentage Distribution | |
| Initial | 2,165 | 98.8% | 22.79 | 1.04% | 3.58 | 0.16% |
| Control | 2,009 | 97.7% | 32.70 | 1.59% | 15.13 | 0.74% |
| Vermicast | 1,918 | 98.1% | 34.38 | 1.76% | 3.71 | 0.19% |
| Carbonized Rice Hull | 1,992 | 98.1% | 35.31 | 1.74% | 2.59 | 0.13% |
| Bagasse Ash | 1,965 | 98.3% | 31.64 | 1.58% | 1.71 | 0.09% |
| Zeolite | 1,893 | 98.7% | 23.74 | 1.24% | 1.07 | 0.06% |
As shown in Table 03, the initial soil sample had the highest Cr found in the residual fraction with 2 165 mg/ kg which comprises 98.8 % of the total Cr, followed by control with 2 009 mg /kg (97.7 %), carbonized rice hull with 1 992 mg/ kg (98.1 %), bagasse ash with 1 965 mg/ kg (98.3%), vermicast with 1 918 mg/ kg (98.1%) while zeolite had the least residual Cr amount with 1 893 mg/ kg (98.7%). The metals found in this phase are not expected to be released in the solution over a reasonable time span under the conditions normally encountered in nature [20] The organic soil amendments were able to increase the amount of Cr bound to organic matter fraction. Carbonized rice hull with 35.31 mg/ kg followed by vermicast with 34.38 mg /kg control with 32.70 mg/ kg, bagasse ash with 31.64 mg/ kg, zeolite with 23.74 mg/ kg and control having the lowest with 22.79 mg/ kg. This is expected since, the ameliorants which are of organic origin contains high amounts of carbon and silica that are known as adsorbent to many impurities including heavy metals. The metals bound to this fraction are assumed to stay in the soil for longer period but may be immobilized by decomposition process [21] For the Cr bound to Fe/Mn oxide, control had the highest with 15.13 mg/ kg followed by vermicast with 3.71 mg/ kg, followed by initial with 3.58 mg/ kg, then carbonized rice hull with 2.58 mg/ kg, then bagasse ash with 1.71 mg/ kg while zeolite had the lowest with 1.07 mg /kg. Within this soil fraction, it is still found to be in non-available form thus not harmful to the environment but with time it can become soil pollutant if no mitigation measures are implemented. Furthermore, there were no detections on the fraction bound to carbonates, in the exchangeable and water fraction which are in the available form. Heavy metals retained in these forms may be potential source of contamination since they could be released if there are changes in the redox status of the soil. Results show that Cr is highly concentrated in the residual fractions in all treatments, with the control having the highest concentration at 2, 008 mg/ kg, followed by CRH (1 992 mg /kg), bagasse ash (1 965 mg/ kg), vermicast (1 917 mg /kg), while zeolite had the least concentration of 1 817 mg/ kg Cr (Table 4). This shows that after the second season, there is continuous decrease in the concentration of Cr in the residual fraction using soil ameliorants. It serves as a very useful guide in the evaluation of long-term potential risk of heavy metal in the environment [22].
Table 04: Total concentration (mg /kg) and percentage distribution of Cr in different fractions as influenced by soil amendments (2018 DS)
| Treatments | Soil Fractions | |||||
| Residual | Bound to OM | Bound to Fe/Mn oxide | ||||
| Total concentration | Percentage Distribution | Total concentration | Percentage Distribution | Total concentration | Percentage Distribution | |
| T1 Control | 2,004 | 98.7% | 23.65 | 1.16% | 2.68 | 0.13% |
| T2 Vermicast | 1,818 | 98.4% | 27.02 | 1.46% | 3.24 | 0.18% |
| T3 Carbonized Rice Hull | 1,991 | 98.6% | 26.60 | 1.32% | 0.86 | 0.04% |
| T4 Bagasse Ash | 1,963 | 98.8% | 21.57 | 1.09% | 1.69 | 0.09% |
| T5 Zeolite | 1,816 | 98.7% | 23.63 | 1.28% | 1.00 | 0.05% |
On Table 04, the levels of Cr on the residual fraction’s ranges from 98.4% in vermicast to 98.8% in bagasse ash. This is followed by bound to organic matter with levels ranging from 1.09% in bagasse ash to 1.46% in vermicast. The last soil fractions where Cr is found are those bound in Fe/Mn oxide where the range is from 0.04% in CRH to 0.18% in vermicast. It can also be noted that Cr bound to organic matter is highest in vermicast with 27.02 mg/ kg followed by CRH with 26.60 mg/ kg, control with 23.65 mg/ kg, zeolite with 23.63 mg /kg, and bagasse ash had the lowest concentrations of 21.57 mg/ kg Cr. For the Cr bound to Fe/Mn oxide, vermicast had the highest with 3.24 mg/ kg followed by control with 2.68 mg/ kg, followed by bagasse ash with 1.69 mg/ kg, then zeolite with 1.00 mg /kg, while CRH had the lowest concentration with 0.86 mg/ kg Cr. Moreover, fractions bound to carbonates, in exchangeable and water soluble, which are in the available forms, are below detectable levels in all treatments. After two seasons, Cr was continuously to reduce in all treatments. Zeolite application resulted in lowest levels of Cr due to its micro-porosity and naturally-occurring hydrated aluminosilicate minerals that act as adsorbents and catalysts while control had the highest levels of Cr in all fractions. Furthermore, for the two cropping seasons, there was no carbon, exchangeable and water fractions were detected for Cr as a mobile form. The levels of Ni in the different soil fractions during 2017 WS is shown in Table 05. It was observed that plots not amended with soil ameliorants had the highest Ni contents distributed among the five fractions. The highest distribution of this element is on the residual fractions and only few from carbonate fractions while water-soluble form is below the detectable levels. Results showed that Ni is highly concentrated in the residual fraction.
Table 05: Concentration (mg/kg) of Ni in different fractions as influenced by soil amendments (2017 WS)
| Treatments | Soil Fractions | |||||
| Residual | OM | Fe/Mn | Carbon | Exchangeable | Water | |
| Initial | 1,155 | 75.71 | 248.79 | 5.10 | 25.75 | – |
| T1 Control | 1,106 | 89.24 | 255.79 | 5.92 | 35.13 | _ |
| T2 Vermicast | 1,127 | 91.80 | 183.93 | 4.81 | 37.19 | – |
| T3 Carbonized Rice Hull | 1,025 | 94.51 | 189.76 | 5.77 | 41.32 | – |
| T4 Bagasse Ash | 1,085 | 88.84 | 159.29 | 4.23 | 36.33 | – |
| T5 Zeolite | 1,064 | 89.91 | 190.00 | 3.53 | 32.01 | – |
Table 06: Concentration (mg/ kg) of Ni in different fractions as influenced by soil amendments (2018 DS)
| Treatments | Soil Fractions | |||||
| Residual | OM | Fe/Mn oxide | Carbon | Exchangeable | Water | |
| T1 Control | 1,033 | 80.16 | 206 | 4.8 | 27.63 | – |
| T2 Vermicast | 967 | 83.19 | 167 | 2.03 | 27.72 | – |
| T3 Carbonized Rice Hull | 972 | 84.47 | 173 | 4.22 | 22.58 | – |
| T4 Bagasse Ash | 763 | 77.81 | 137 | 2.74 | 20.42 | – |
| T5 Zeolite | 822 | 72.24 | 150 | 2.83 | 14.57 | – |
Table 06 shows significant concentrations of Ni in the residual and bound to Fe-Mn oxide fractions followed by bound to OM, exchangeable and lastly bound to carbonates fraction. Results implied that Ni was highly concentrated in the residual fraction with the control having the highest at 1 033 mg /kg, followed carbonized rice hull at 972 mg/ kg, vermicast at 967 mg/ kg, zeolite at 822 mg/ kg, while bagasse ash had the least concentration with 763 mg /kg Ni. Nickel bound to organic matter is highest in carbonized rice hull with 84.47 mg /kg followed by vermicast with 83.19 mg /kg, then control with 80.16 mg/ kg, bagasse ash with 77.81 mg /kg, the least is zeolite with 72.24 mg/ kg. The soil amendments of organic origin also had the highest levels of Ni in the organic bound fraction. For the Ni bound to Fe-Mn oxide, control had the highest with 206 mg/ kg, followed by CRH with 173 mg/ kg, then vermicast with 167 mg/ kg, zeolite with 150 mg/ kg, while bagasse ash had the lowest at 137 mg /kg. Ni levels found in the carbonate fraction was highest in the control (4.80 mg/ kg), followed by CRH (4.22 mg/ kg), zeolite (2.83 mg/ kg), bagasse ash (2.74 mg /kg), while vermicast has the least concentration of Ni at 2.03mg/ kg. The distribution of Ni during 2018 DS using soil ameliorants in decreasing order are as follows: residual>Fe/Mn> OM>exchangeable>carbon. For the exchangeable fraction, vermicast has the highest concentration with 27.72 mg /kg, then control with 27.63 mg/ kg, carbonized rice hull with 22.58 mg/ kg bagasse ash with 20.42 mg/ kg, and lowest level is zeolite with 14.57 mg/ kg. There was no Ni detected in the water-soluble fraction which is the most available and hazardous form. However, among the six fractions, only the exchangeable and those bound to carbonate fractions are in available forms. After two cropping seasons (2017 WS and 2018 DS), the use of different soil amendments like vermicast, carbonized rice hull, bagasse ash and zeolite have reduced the levels of Cr and Ni in the soil and have shown potential for remediation to both Cr and Ni. It was observed that the total Cr and Ni in the soil fractions are relatively high in all treatments. Cr (> 1,900 mg kg-1 2017 WS; >1,800 mg/ kg 2018 DS) was also found to be of higher concentration than Ni (>1,300 mg /kg 2017 WS; >1,000 mg/ kg 2018 DS). It was observed that after 2017 WS, zeolite was the most effective in reducing total Cr by more than 12% and after 2018 DS by nearly 16%. For Ni, carbonized rice hull was most effective by reducing more than 10% at 2017 WS, and more than 33% was reduced using bagasse ash during 2018 DS. This shows that ameliorants containing high amounts of carbon has been most effective in reducing total Ni. For Cr and Ni distribution in the different soil fractions, Cr was observed in three fractions. More than 97% in all treatments can be found in the residual fraction. Nearly 1% was found in the organic fraction and less than 0.2% was found in the Fe/Mn fraction. Moreover, Cr is found to be higher in total concentration content, and found only in the soil fractions that are considered to be most stable, unavailable and immobile. Ni on the other hand has lower total concentration content, it was observed that Ni was present in four out of the six fractions. Greater fractions were bound to residual with more than 74% and around 6% is found in the organic fraction, more than 12% was observed in bound to Fe-Mn fraction and less than 2% in the exchangeable fraction. This means that nickel poses more hazardous impact to the environment and the crops planted, since, those that are found in the carbonate and exchangeable fractions are most mobile and readily available form of contaminants. The most reactive, most mobile and most potentially available/bioavailable fractions are found in water soluble, exchangeable and carbonate fractions (water soluble + exchangeable + carbonate). The metals in these fractions consist of those that can be accessed by man through ingestion and are usually considered as an anthropogenic origin. The metals in these phases are weak or loosely bound to soil components and the relative index mobility of these metals was calculated as mobility factor (MF) using the following equation.
Table 07: Mobility factor of Cr and Ni for two cropping seasons as influenced by soil amendments
| Cr 2017 WS | Ni 2017 WS | Cr 2018 DS | Ni 2018 DS | |
| Initial | – | 2.00 | – | _ |
| T1 Control | – | 2.70 | – | 2.45 |
| T2 Vermicast | – | 2.90 | – | 2.40 |
| T3 Carbonized Rice Hull | – | 3.40 | – | 2.20 |
| T4 Bagasse Ash | – | 2.95 | – | 2.40 |
| T5 Zeolite | – | 2.57 | – | 1.66 |
Results show a consistent immobility effect of zeolite to Ni in both wet and dry season (Table 07). This implies that zeolite has displays an excellent affinity for metal cations and adsorption of catalytic properties. In addition, cations of sodium, calcium, and potassium that are typically present in the channels of the zeolite structure can be replaced by other metal cations, including lead, cadmium, zinc, copper, nickel, iron, and manganese [23]; found out the potential use of Philippine natural zeolites for treating acid mine drainage (Cu, Ni, As, Pb and Zn) in Rapu- Rapu. The authors reported that the heavy metal uptake of zeolite was 35.88% for Cu; 35.36% for Ni; 95.79% for as; 99.03 for Pb; and 33.91% for Zn. Addition of this mineral increases pH, that causes precipitation of hydrous ferric oxides and decrease in dissolved metal concentrations [20] Thus, it has been widely used for its effective, efficient, adsorptive property, low cost and locally available for mitigating heavy metals. Generally, the mobility factor in all treatments in two cropping seasons were less than 5 %. This implies that the mobility and biological availability of Nickel is relatively low which indicate low potential risk to the environment. Furthermore, no mobile Cr was detected for the two cropping seasons. Grain yield (t ha-1). Grain yield is the function of biomass accumulation during ripening and translocation of biomass accumulated in the grains before flowering [36]. Maximum grain yield of 6.6 – 6.8 t ha-1 was observed in plots treated with 10 t ha-1 vermicast as shown in Table 8. The plants treated with inorganic fertilizer alone (farmer’s practice) had the lowest yield of 3.8 – 4.1 t ha-1. Results also revealed that addition of soil amendments at 10 t /ha significantly increased the yield. The increase in grain yield could be due to the increase in yield attributes. Similar results were reported. Providing additional evidence that organic fertilizers have a significant influence on growth and productivity in lowland rice. Organic soil amendments can be a good supplement to inorganic fertilizer to produce better growth and yield. All the treatments applied with 10t ha-1 organic material resulted in significant improvement in the growth and productivity of rice. From the economic point of view farmers, can use the combination of organic fertilizer and reduced rates of inorganic fertilizers to boost the yield of rice as well as to maintain and improve soil health. Return on investment (ROI) describes the ratio of net income with total expenses. Table 8 shows the highest ROI value of 124% (WS) and 130 % (DS) were derived from the application of Farmers practice plus 10t/ha bagasse ash due to its cheaper cost with great impact on crop productivity. This parameter indicates the best fertilization combination to gain profit in farming without compromising the natural resources particularly the soil. In economic view, this means that as much as 124 to 130 pesos can be gained for every peso investment when using this treatment. The lowest ROI value of -30% (WS) and -22% (DS) were recorded from the application of Farmers practice plus 10t/ha zeolite, indicating that among the treatments, this was the least profitable due to its expensive cost at 10 pesos per kilo. Application of zeolite and organic soil amendments (10 t/ha) significantly increased grain yield over the control (Table 8). Higher grain yield was obtained during 2018 DS due to higher solar radiation which has a significant role in improving crop productivity.
Table 08: Yield components of rice (kg ha-1) as affected by different soil amendments for two cropping seasons
| Treatment | Grain Yield (kg/ha) | ROI (%) | ||
| WS (2007) | DS (2008) | WS (2007) | DS (2008) | |
| ** | ** | |||
| Control | 3800 e | 4110 e | 65.0 | 74.0 |
| Vermicast | 6600 a | 6884 a | -2.0 | 1.0 |
| Carbonized rice hull | 5100 d | 5410 d | 29.0 | 34.0 |
| Bagasses ash | 5400 c | 5670 c | 124.0 | 130.0 |
| Zeolite | 5500 b | 6164 b | -30.0 | -22.0 |
| Grand Mean | 5280 | 5650 | 37.0 | 43.0 |
**=highly significant 1In a column, means followed by the same letter(s) are not significantly different at 5% level by LSD.
* Computed based on the prevailing price of palay in the study area @18 kg-1.
** Return on investment ha-1.
Table 09 shows the bioaccumulation factor (BAF) of Cr and Ni in rice grain at harvest as influenced by the different soil amendments. For Cr, BAF was lowest with zeolite (0.401), followed by vermicast (0.473), carbonized rice hull (0.504), bagasse ash (0.562) and control (0.748). For Ni, BAF was lowest with carbonized rice hull (0.484), followed by zeolite (0.401), vermicast (0.504), bagasse ash (0.527) and control (0.711). All BAF values were below 1 indicating that rice absorbs but not stores Cr and Ni. The amount of Cr and Ni transferred from the soil to the plant is one of the key elements in the human exposure to metals via the food chain [40]. Translocation factors are also very low, particularly with the application of soil amendments. For Cr translocation factor are 0.005 for carbonized rice hull and bagasse ash, 0.006 for zeolite and vermicast which are significantly lower than TF of the control (0.016). For Ni lowest TF was for zeolite (0.006) followed by carbonized rice hull (0.009), bagasse ash (0.010), vermicast (0.012) and control (0.034).
Table 09: Bio-accumulation and translocation factor in rice as affected by soil amendments in 2017 WS
| Bio-accumulation factor | Translocation factor | |||
| Treatments | Cr(ppm)** | Ni(ppm)** | Cr(ppm) * | Ni(ppm)** |
| T1-Control | 0.748 a | 0.711 a | 0.016 a | 0.034 a |
| T2-Vermicast | 0.473 d | 0.504 c | 0.006 b | 0.012 b |
| T3-Carbonnized rice hull | 0.504 c | 0.484 d | 0.005 c | 0.009 d |
| T4- Bagasse ash | 0.562 b | 0.527 b | 0.005 c | 0.01 c |
| T5- Zeolite | 0.401 | 0.488 | 0.006 b | 0.006 |
*= significant; **=highly significant
1In a column, means followed by the same letter(s) are not significantly different at 5% level by LSD
Table 10 shows the transfer of metals from the soil to the plant is one of the key elements of human exposure to metals via the food chain [19]. When the BAF is <1 or =1, it indicates that the plant only absorbs but does not store heavy metals; when BAF is >1, it is an indication that the plant stores metals. The BAF of these two metals were less than 1 indicating that the plant only absorbs metals. It was found out that farmers practice had the highest amount of accumulated metals (0.24 ppm Cr and 0.29 ppm Ni) compared to plants added with soil amendments like vermicast, carbonized rice hull, bagasse ash and zeolite show below detection limit on Cr in rice grains. Ni was bio-accumulated more in the control with 0.29 ppm while vermicast and bagasse ash had 0.25 ppm and below detection limit for zeolite and carbonized rice hull. On the other hand, translocation factor is one of the key elements of human exposure to metals via the food chain. It was observed that Cr is below detection limit in treatments with vermicast, carbonized rice hull, bagasse ash, and zeolite while the translocated amount of nickel was below detection limit to those treatments with carbonized rice hull and zeolite. Other treatments had lower translocated amount of 0.02 in both vermicast and bagasse ash while farmers practice had 0.04 Cr and 0.03 ppm Ni.
Table 10: Bio-accumulation and translocation factor in rice as affected by soil amendments in 2018 DS.
| Bio-accumulation factor * | Translocation factor * | |||
| Treatments | Cr | Ni | Cr | Ni |
| T1-Control | 0.24 | 0.29 | 0.04 | 0.03 |
| T2-Vermicast | BDL | 0.25 | BDL | 0.02 |
| T3-Carbonized rice hull | BDL | BDL | BDL | BDL |
| T4- Bagasse ash | BDL | 0.25 | BDL | 0.02 |
| T5- Zeolite | BDL | BDL | BDL | BDL |
4. CONCLUSION
Based on the results obtained, the following conclusions are drawn. The study site is contaminated with Cr and Ni due to its naturally occurring mineral from parent material and the presence of mining activities. In this study, Cr levels in the various chemical fractions is in decreasing order as follows: residual > bound to organic matter > bound to Fe/Mn oxide only while Ni occurs in the following fractions in decreasing order: residual > bound Fe/Mn oxide >bound to organic matter > exchangeable >bound to carbonate. Between the readily available fraction and the unavailable (residual) fraction or trace metals, there exists a number of geochemical phases that may potentially release trace metals, these are fractions from Carbonate, Fe/Mn oxide and organic matter bound. They constitute most important pools of potentially available trace metals which their released depend on existence of suitable physiochemical factors such as Redox potential, Temperature and pH of the medium. However, bioavailability of metals in the geochemical fractions decreases as follow; water>exchangeable > carbonate > Fe/Mn oxide> organic matter > residual. Moreover, in accordance with who defined a model for heavy metal association with geochemical fractions the first four fractions mentioned above are considered as potentially available fractions (readily available for plant uptake and biota bio- accumulation). The mobility factor is only for Ni since there was no reading for Cr in most potentially available/bioavailable fractions. Zeolite tend to be immobile with Ni since this treatment shows low mobility factor in both wet and dry seasons. However, during dry season the farmer’s practice or no soil amendments show higher mobility factor for Ni indicating the presence of Ni in available and hazardous form to the environment. Generally, all treatments with soil amendments were obtained higher yield compared to farmers practice in both wet and dry season. Highest yield was obtained from treatments with vermicast however, highest return on investment was obtained in plots added with bagasse ash with 124% (WS) and 130% (DS) due to its cheaper cost while lowest ROI was obtained in plots added with zeolite with -30% (WS) and -22% (DS) because the material cost at 10.00 kg-1 at 10t ha-1 recommendation rate. Plants applied with zeolite and carbonized rice hull show below detection limit (BDL) in both bio-accumulation and translocation factor of Cr and Ni after two cropping seasons. All soil amendments display its affinity to immobilize Cr while carbonized rice hull and zeolite are effective also in mitigating Ni.
REFERENCES
- Chad, F. (2025). Mechanisms of Chromium and Nickel Uptake by Vetiver Grass in Contaminated Agricultural Soils.
- Nguyen, T. T. T., Vu, T. A. N., Nguyen, D. P., Nguyen, V. H. N., Pham, T. T. H., Truong, T. T., … & Vuong, T. X. (2024). Lead and zinc chemical fraction alterations in multi-metal contaminated soil with pomelo peel biochar and biochar/apatite incubation. Materials Research Express, 11(4), 045602.
- Muhammad Rizwan, Junaid Zaheer, Muhammad Naveed Tahir, Muhammad Ansar & Hurairah Ejaz. Pakistan’s Wheat Production and the Effects of Climate Change. Dinkum Journal of Natural & Scientific Innovations, 2(09):514-526.
- Daripa, A., Chattaraj, S., Malav, L., Ray, P., Sharma, R., Mohekar, D. S., … & Patil, N. G. (2023). Risk assessment of agricultural soils surrounding an iron ore mine: A field study from Western Ghat of Goa, India. Soil and Sediment Contamination: An International Journal, 32(5), 570-590.
- Yang, J. M., Chen, H. L., Wang, X., Guan, D. X., Ji, X. H., Xie, Y. H., … & Qin, Q. B. (2025). Toward safe rice production in As-Cd co-contaminated paddy soils: Biogeochemical mechanisms and remediation strategies. Critical Reviews in Environmental Science and Technology, 55(1), 1-24.
- Muhammad Rizwan, Asim Masood, Fatima Zaheer, Abdul Saboor, Hurairah Ejaz & Kamran Afzal. China’ Big Agri-Product Consumption Market: How Pakistan can access it?. Dinkum Journal of Natural & Scientific Innovations, 2(09):527-530.
- Guan, Z., Wei, R., Liu, T., Li, J., Ao, M., Sun, S., … & Qiu, R. (2023). Water Management Impacts on Chromium Behavior and Uptake by Rice in Paddy Soil with High Geological Background Values. Toxics, 11(5), 433.
- Kukowska, S., & Szewczuk-Karpisz, K. (2024). Biochar and Zeolite Uses in Improving Immobilization of Nutrients and Pollutants in Soils. Separation & Purification Reviews, 1-24.
- Afzal, M., Muhammad, S., Tan, D., Kaleem, S., Khattak, A. A., Wang, X., … & Tan, Z. (2024). The effects of heavy metal pollution on soil nitrogen transformation and rice volatile organic compounds under different water management practices. Plants, 13(6), 871.
- Massas, I., Kairis, O., Gasparatos, D., Ioannou, D., Vatougios, D., & Zafeiriou, I. (2023). Impaired soil health in agricultural areas close to Fe-Ni mines on Euboea Island, Greece, caused by increased concentrations of potentially toxic elements, and the associated impacts on human health. Environments, 10(9), 150.
- Zhang, J., Yang, L., Liu, Y., Xing, M., Wu, Y., & Bing, H. (2024). Pollution and mobility of heavy metals in the soils of a typical agricultural zone in eastern China. Environmental geochemistry and health, 46(3), 91. Zhang, J., Yang, L., Liu, Y., Xing, M., Wu, Y., & Bing, H. (2024). Pollution and mobility of heavy metals in the soils of a typical agricultural zone in eastern China. Environmental geochemistry and health, 46(3), 91.
- Oghenenyoreme Eyankware, M., Akakuru, O. C., Igwe, E. O., Olajuwon, W. O., & Ukor, K. P. (2024). Pollution indices, potential ecological risks and spatial distribution of heavy metals in soils around Delta State, Nigeria. Water, Air, & Soil Pollution, 235(7), 452.
- Siddique, A. B., Islam, M. R., Shahid, M., Billah, M. M., Naidu, R., & Rahman, M. M. (2024). Root Iron Plaque Formation and Cadmium Accumulation in Paddy Rice: A Literature-Based Study. Cadmium Toxicity in Water: Challenges and Solutions, 265-297.
- Umeobi, E. C., Azuka, C. V., Ofem, K. I., Obite, S. U., Ezea, C. A., Abraham, I. I., … & Ezeaku, P. I. (2024). Evaluation of soil pollution effects on maize (Zea mays) at selected Pb–Zn and limestone mine sites in Ebonyi State, Southeastern Nigeria. Environmental Monitoring and Assessment, 196(8), 768.
- Sharma, U. C., Datta, M., & Sharma, V. (2025). Chemistry, Microbiology, and Behaviour of Acid Soils. In Soil Acidity: Management Options for Higher Crop Productivity (pp. 121-322). Cham: Springer Nature Switzerland.
- Saeed, M., Ilyas, N., Bibi, F., Shabir, S., Mehmood, S., Akhtar, N., … & Eldin, S. M. (2023). Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview. Nanotechnology Reviews, 12(1), 20230156.
- Lovynska, V., Bayat, B., Bol, R., Moradi, S., Rahmati, M., Raj, R., … & Montzka, C. (2024). Monitoring heavy metals and metalloids in soils and vegetation by remote sensing: a review. Remote Sensing, 16(17), 3221.
- Barman, A., Tiwari, A. K., & Dhar, P. (2024). Breath of the future: advancing air pollution solutions through innovative technologies and global collaboration. A Comprehensive Exploration of Soil, Water, and Air Pollution in Agriculture, 366.
- Kousar, S., Ahmad, D., Khattak, W. A., Anwar, M. M., Majeed, M., Shah, G. M., & Muhammad, M. (2025). Environmental resilience: navigation of lead and beneficial elements in soil. In Beneficial Elements for Remediation of Heavy Metals in Polluted Soil (pp. 221-240). Elsevier.
- MUHAMMAD AFZAL, S. M., Kaleem, S., Khattak, A. A., Wang, X., Chen, X., Fang, M. L., … & Tan, Z. (2024). The Effects of Heavy Metals Pollution on Nitrogen Transformation and Volatile Organic Compounds in Paddy Soil under Different Water Management Practices.
- Bedadi, B., Beyene, S., Erkossa, T., & Fekadu, E. (2023). Soil management. In The Soils of Ethiopia (pp. 193-234). Cham: Springer International Publishing.
- Han, X., Wang, F., Zhao, Y., Meng, J., Tian, G., Wang, L., & Liang, J. (2023). Recycling of iron ore tailings into magnetic nanoparticles and nanoporous materials for the remediation of water, air and soil: a review. Environmental Chemistry Letters, 21(2), 1005-1028.
- Kamewada, K., & Ooshima, M. (2024). Modeling and simulation of sulfur availability in paddy soils under reducing environment considering heavy metal content. Soil Science and Plant Nutrition, 70(4), 283-294.
- Kibret, K., Abera, G., & Beyene, S. (2023). Soils and Society. In The Soils of Ethiopia (pp. 257-281). Cham: Springer International Publishing.
- Bolan, N., & Kirkham, M. B. (Eds.). (2023). Soil Constraints and Productivity. CRC Press.
- Sharma, U. C., Datta, M., & Sharma, V. (2025). Managing Soil Acidity. In Soil Acidity: Options for Higher Crop Productivity(pp. 427-522). Cham: Springer Nature Switzerland.
Publication History
Submitted: October 31, 2024
Accepted: November 22, 2024
Published: March 31, 2025
Identification
D-0412
DOI
https://doi.org/10.71017/djnsi.4.3.d-0412
Citation
Venus O. Saz & Hamsha R. Saz (2025). Application of Soil Amendments on the Chemical Fractions of Chromium and Nickel in Lowland Rice Soils as Affected by Mining Activities Dinkum Journal of Natural & Scientific Innovations, 4(03):195-209.
Copyright
© 2025 The Author(s).


